Marine sponges are multicellular and primitive animals that potentially represent a wealthy source of novel drugs. The genus Acanthella (family Axinellidae) is renowned to produce various metabolites with various structural characteristics and bioactivities, including nitrogen-containing terpenoids, alkaloids, and sterols. Different metabolites were separated and characterized from different species of this genus using various spectroscopic and chromatographic techniques. The isolated metabolites are categorized according to their chemical classes into sesquiterpenes, diterpenes, alkaloids, steroid compounds, and others. Additionally, their reported biosynthetic and synthetic studies are also highlighted whenever applicable.
- Acanthella
- Axinellidae
- nitrogen-containing terpenoids
- Life below water
1. Sesquiterpenes
| Compound Name | Mol. Wt. | Mol. Formula | Species | Sampling Locations | Ref. |
|---|---|---|---|---|---|
| Aromadendrane-type sesquiterpenes | |||||
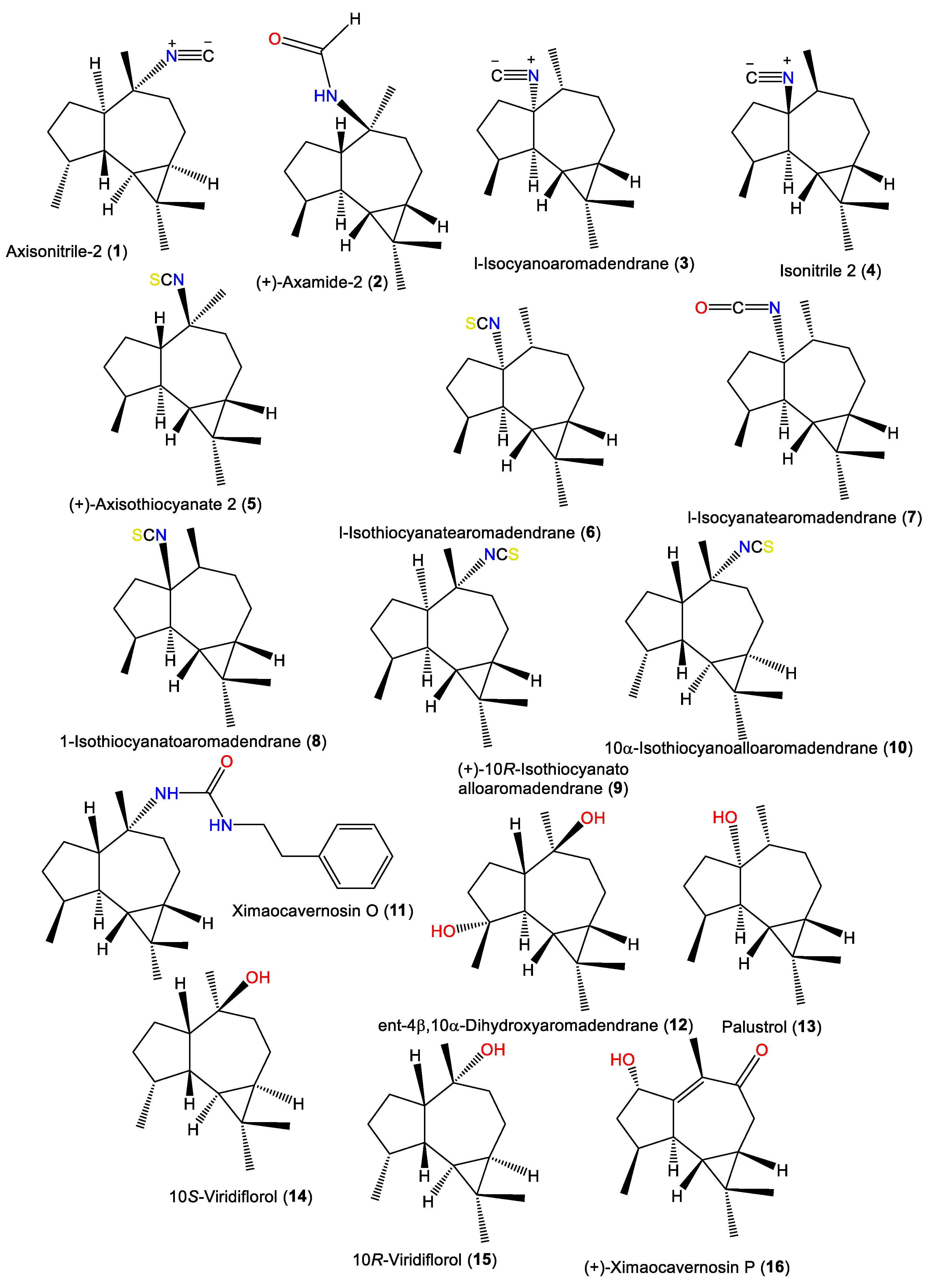
| Axisonitrile-2 (1) | 231 | C16H25N | A. cavernosa | Hachijo-Jima Island, Japan | [3] |
| (+)-Axamide 2 (2) | 249 | C16H27NO | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| l-Isocyanoaromadendrane (3) | 231 | C16H25N | A. acuta | Near Banyuls, France | [5][6] |
| - | - | A. pulcherrima | Weed Reef, Darwin, Australia | [6] | |
| - | - | A. acuta | Near Banyuls, France | [7] | |
| - | - | A. cavernosa | Tani’s Reef, Mooloolaba, Australia | [8] | |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [9] | |
| Isonitrile 2 (4) | 231 | C16H25N | A. acuta | Bay of Naples, southern Italy | [10] |
| (+)-Axisothiocyanate 2 (5) | 263 | C16H25NS | A. cavernosa | Hachijo-jima Island, Japan | [11] |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [12] | |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [9] | |
| l-Isothiocyanatearomadendrane (6) | 263 | C16H25NS | A. acuta | Near Banyuls, France | [7] |
| - | - | A. cavernosa | Tani’s Reef, Mooloolaba, Australia | [8] | |
| 1-Isocyanatearomadendrane (7) | 247 | C16H25NO | A. acuta | Near Banyuls, France | [7] |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [9] | |
| 1-Isothiocyanatoaromadendrane (8) | 263 | C16H25NS | A. acuta | Bay of Naples, southern Italy | [10] |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [9] | |
| (+)-10R-Isothiocyanatoalloaromadendrane (9) | 263 | C16H25NS | A. cavernosa | Hachijo-jima Island, Japan | [11] |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [12] | |
| - | - | Acanthella sp. | Yalong Bay, Hainan, China | [13] | |
| - | - | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] | |
| 10α-Isothiocyanoalloaromadendrane (10) | 263 | C16H25NS | A. pulcherrima | Weed Reef, Darwin, Australia | [6] |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [9] | |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [12] | |
| - | - | A. cavernosa | Tani’s Reef, Mooloolaba, Australia | [8] | |
| Ximaocavernosin O (11) | 368 | C24H36N2O | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
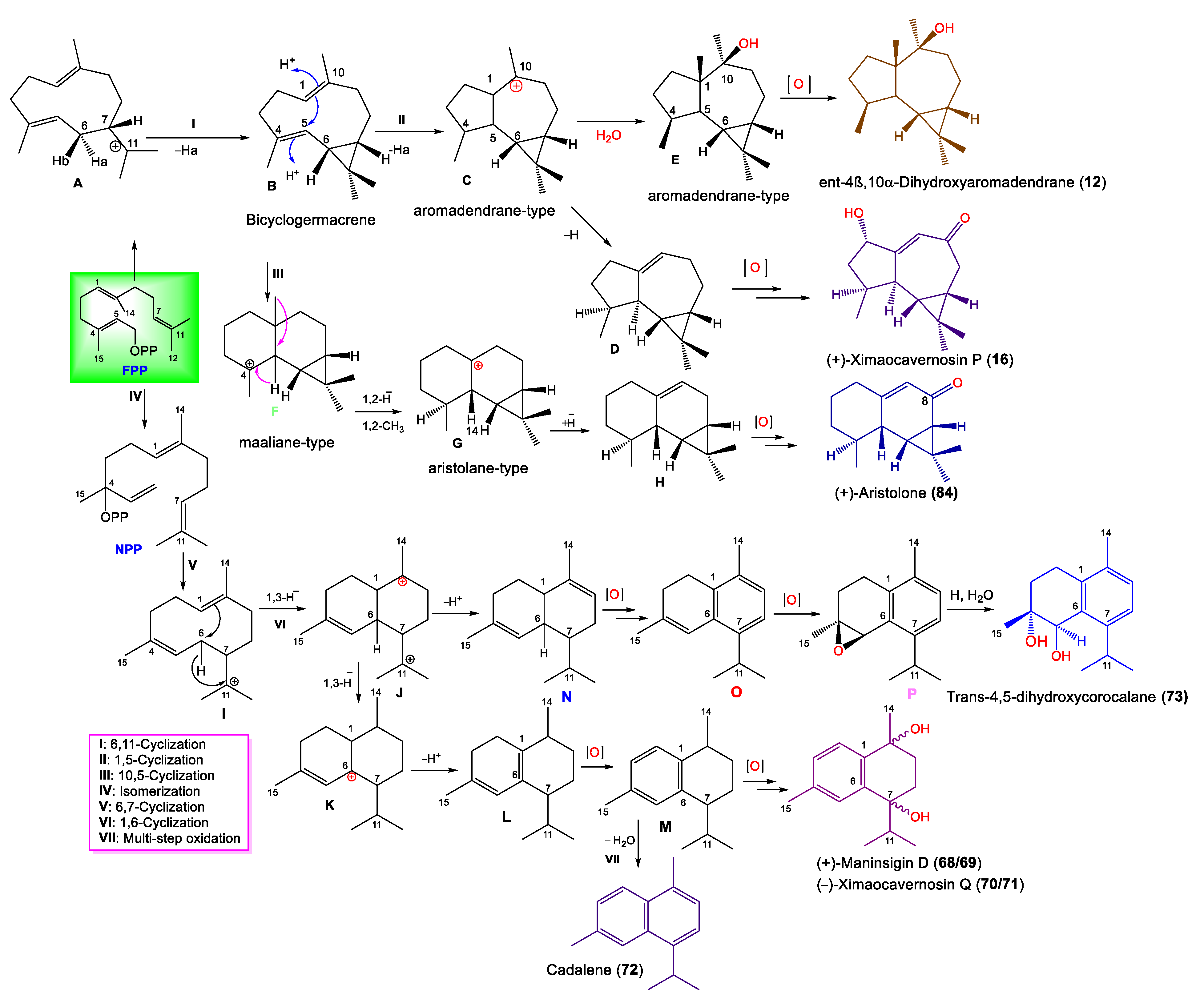
| ent-4β,10α-Dihydroxyaromadendrane (12) | 238 | C15H26O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [14] |
| Palustrol (13) | 222 | C15H26O | A. acuta | Near Banyuls, France | [5] |
| - | - | A. acuta | Near Banyuls, France | [7] | |
| 10S-Viridiflorol (14) | 222 | C15H26O | A. cavernosa | Hachijo-jima Island, Japan | [11] |
| 10R-Viridiflorol (15) | 222 | C15H26O | A. cavernosa | Hachijo-jima Island, Japan | [11] |
| (+)-Ximaocavernosin P (16) | 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [14] |
| Spiroaxane-type sesquiterpenes | |||||
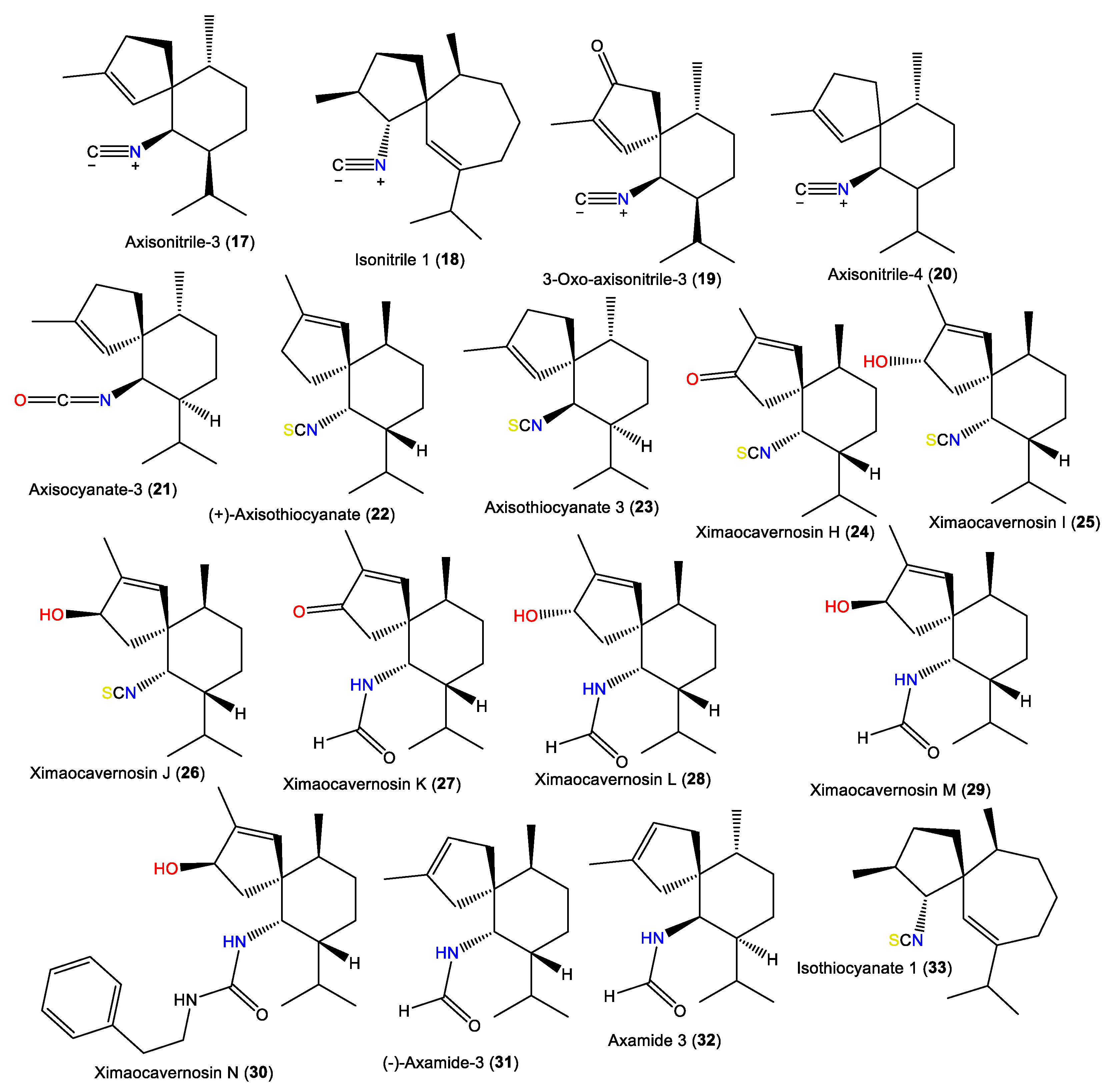
| Axisonitrile-3 (17) | 231 | C16H25N | A. acuta | Near Banyuls, France | [5] |
| - | - | A. cavernosa | Thailand | [15] | |
| - | - | A. klethra | Pelorus Island, Queensland, Australia | [16] | |
| - | - | A. klethra | Vicinities of Phantom and Pelom Islands, Queensland, Australia | [17] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [2] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] | |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [12] | |
| - | - | A. cavernosa | Tani’s Reef, Mooloolaba, Australia | [8] | |
| Isonitrile 1 (18) | 245 | C17H27N | A. acuta | Bay of Naples, southern Italy | [10] |
| 3-Oxoaxisonitrile-3 (19) | 245 | C16H23NO | Acanthella sp. | Ximao Sea, Hainan, China | [12] |
| Axisonitrile-4 (20) | 231 | C16H25N | A. acuta | Sidi Elghdamssi island, Monastir region, Tunisia | [19] |
| Axisocyanate-3 (21) | 247 | C16H25NO | A. cavernosa | Tani’s Reef, Mooloolaba, Australia | [8] |
| (+)-Axisothiocyanate (22) | 263 | C16H25NS | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| Axisothiocyanate 3 (23) | 263 | C16H25NS | A. klethra | Pelorus Island, Queensland, Australia | [16] |
| - | - | A. klethra | Vicinities of Phantom and Pelom Islands, Queensland, Australia | [17] | |
| - | - | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [20] | |
| - | - | A. cavernosa | Hachijo-jima Island, Japan | [11] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [2] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] | |
| Ximaocavernosin H (24) | 277 | C16H22NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| Ximaocavernosin I (25) | 279 | C16H25NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| Ximaocavernosin J (26) | 279 | C16H25NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| Ximaocavernosin K (27) | 263 | C16H25NO2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| Ximaocavernosin L (28) | 265 | C16H27NO2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| Ximaocavernosin M (29) | 265 | C16H27NO2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| Ximaocavernosin N (30) | 368 | C24H36N2O | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| (–)-Axamide 3 (31) | 249 | C16H27NO | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| Axamide 3 (32) | 249 | C16H27NO | A. cavernosa | Hachijo-jima Island, Japan | [11] |
| Isothiocyanate 1 (33) | 277 | C17H27NS | A. acuta | Bay of Naples, southern Italy | [10] |
| Eudesmane-type sesquiterpenes | |||||
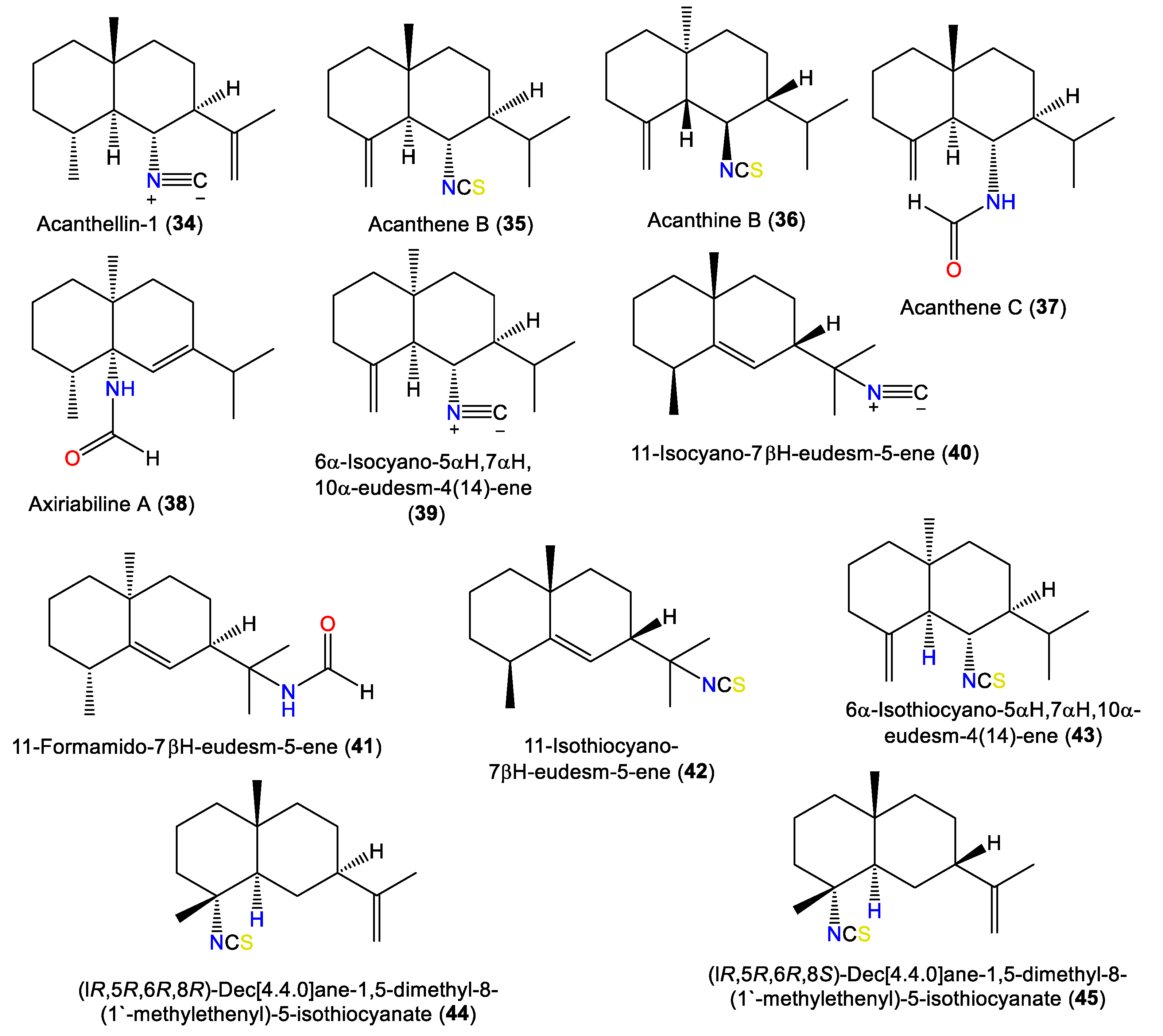
| Acanthellin-1 (34) | 231 | C16H25N | A. acuta | Bay of Naples, southern Italy | [1] |
| - | - | A. acuta | Near Banyuls, France | [5] | |
| - | - | A. acuta | Bay of Taranto near Porto Cesareo, Southern Italy | [21] | |
| - | - | A. acuta | Sidi Elghdamssi Island, Monastir region, Tunisia | [19] | |
| Acanthene B (35) | 263 | C16H25NS | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [20] |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [12] | |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [9] | |
| Acanthine B (36) | 263 | C16H25NS | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| Acanthene C (37) | 249 | C16H27NO | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [20] |
| Axiriabiline A (38) | 249 | C16H27NO | A. cavernosa | Xidao Island, Hainan, China | [22] |
| 6α-Isocyano-5αH,7αH,10α-eudesm-4(14)-ene) (39) | 231 | C16H25N | A. acuta | Bay of Taranto near Porto Cesareo, Southern Italy | [21] |
| - | - | A. acuta | Bay of Naples, southern Italy | [10] | |
| 11-Isocyano-7βH-eudesm-5-ene (40) | 231 | C16H25N | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [20] |
| 11-Formamido-7βH-eudesm-5-ene (41) | 249 | C16H27NO | A. cavernosa | Xidao Island, Hainan, China | [22] |
| 11-Isothiocyano-7βH-eudesm-5-ene (42) | 263 | C16H25NS | A. pulcherrima | Weed Reef, Darwin, Australia | [6] |
| - | - | A. klethra | Pelorus Island, Queensland, Australia | [16] | |
| - | - | A. klethra | Vicinities of Phantom and Pelom Islands, Queensland, Australia | [17] | |
| - | - | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [20] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [2] | |
| 6α-Isothiocyano-5αH,7αH,10α-eudesm-4(14)-ene (43) | 263 | C16H25NS | A. acuta | Bay of Taranto near Porto Cesareo, Southern Italy | [21] |
| - | - | A. acuta | Bay of Naples, southern Italy | [10] | |
| - | - | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [20] | |
| (lR,5R,6R,8R)-Dec[4.4.0]ane-1,5-dimethyl-8-(1′-methylethenyl)-5-isothiocyanate (44) | 263 | C16H25NS | A. klethra | Pelorus Island, Queensland, Australia | [16] |
| - | - | A. klethra | Vicinities of Phantom and Pelom Islands, Queensland, Australia | [17] | |
| - | - | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [20] | |
| (lR,5R,6R,8S)-Dec[4.4.0]ane-1,5-dimethyl-8-(1′-methylethenyl)-5-isothiocyanate (45) | 263 | C16H25NS | A. klethra | Pelorus Island, Queensland, Australia | [16] |
| - | - | A. klethra | Vicinities of Phantom and Pelom Islands, Queensland, Australia | [17] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] | |
| Cadinene-type sesquiterpenes | |||||
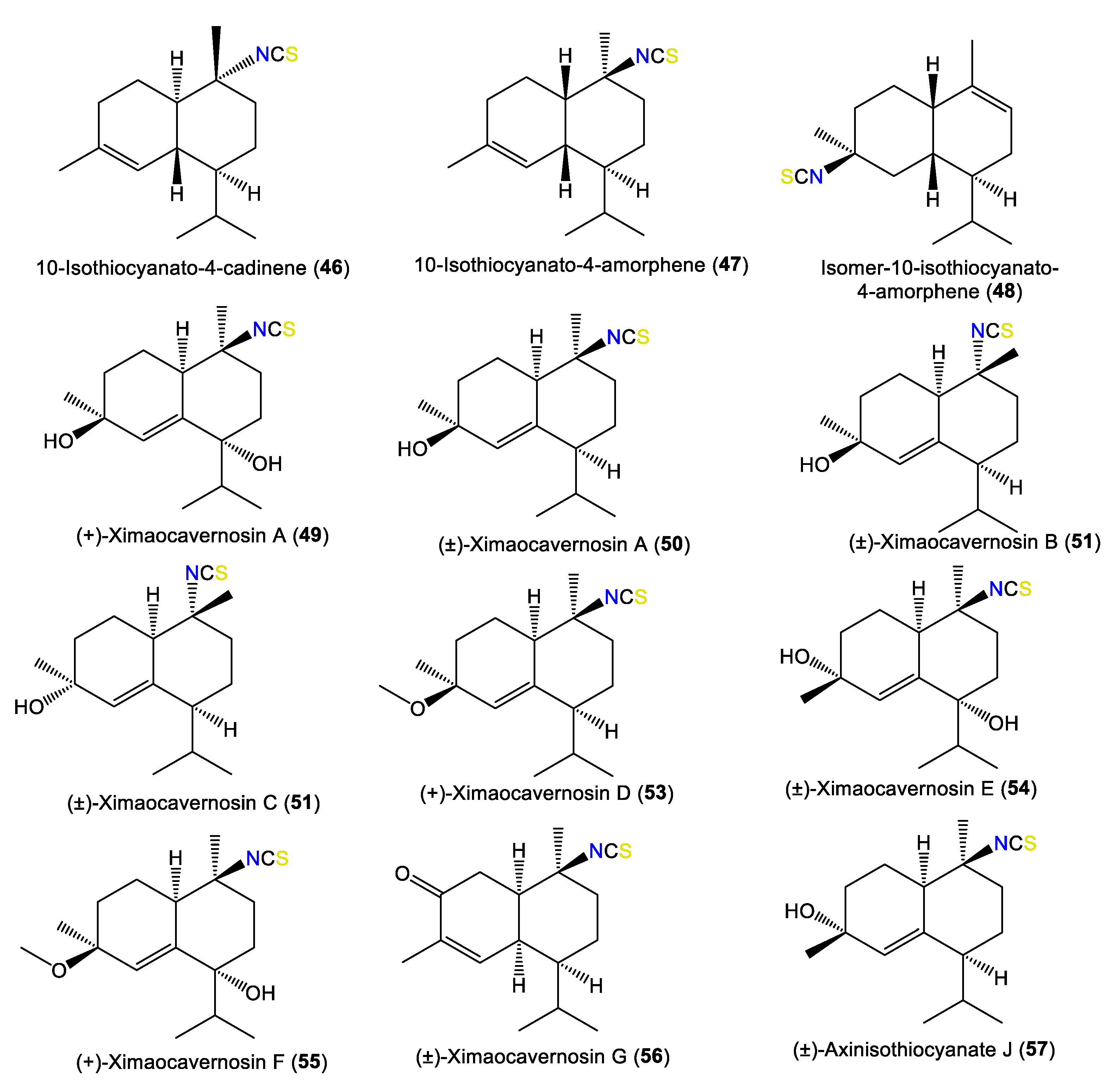
| 10-Isothiocyanato-4-cadinene (46) | 263 | C16H25NS | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [9] | |
| 10-Isothiocyanato-4-amorphene (47) | 263 | C16H25NS | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] |
| - | - | A. cavernosa | Several locations off the Japanese coast | [23] | |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [12] | |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [9] | |
| Isomer-10-isothiocyanato-4-amorphene (48) | 263 | C16H25NS | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] |
| (+)-Ximaocavernosin A (49) | 295 | C16H25NO2S | A. cavernosa | Coast of Ximao Island, Hainan, China | [14] |
| (±)-Ximaocavernosin A (50) | 279 | C16H25NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| (±)-Ximaocavernosin B (51) | 279 | C16H25NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| (±)-Ximaocavernosin C (52) | 279 | C16H25NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| (+)-Ximaocavernosin D (53) | 293 | C17H27NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| (±)-Ximaocavernosin E (54) | 295 | C16H25NO2S | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| (+)-Ximaocavernosin F (55) | 309 | C17H27NO2S | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| (±)-Ximaocavernosin G (56) | 277 | C16H23NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| (±)-Axinisothiocyanate J (57) | 279 | C16H25NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
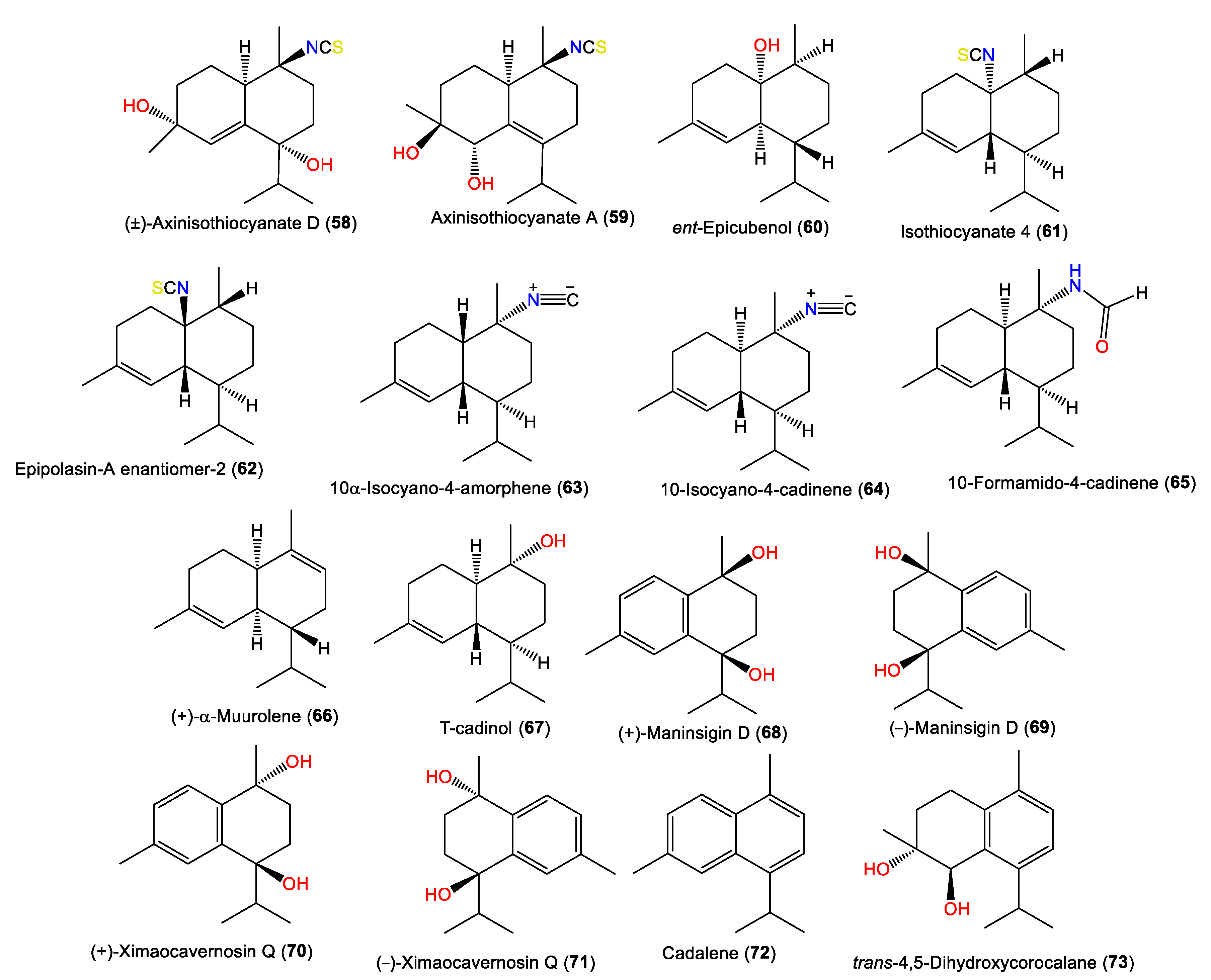
| (±)-Axinisothiocyanate D (58) | 295 | C16H25NO2S | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| Axinisothiocyanate A (59) | 295 | C16H25NO2S | A. cavernosa | Coast of Ximao Island, Hainan, China | [4] |
| ent-Epicubenol (60) | 222 | C15H26O | A. pulcherrima | Weed Reef, Darwin, Australia | [6] |
| Isothiocyanate 4 (61) | 263 | C16H25NS | A. pulcherrima | Weed Reef, Darwin, Australia | [6] |
| Epipolasin-A enantiomer-2 (62) | 263 | C16H25NS | A. pulcherrima | Weed Reef, Darwin, Australia | [6] |
| 10α-Isocyano-4-amorphene (63) | 231 | C16H25N | A. cavernosa | Hachijo-Jima Island, Japan | [3] |
| - | - | A. cavernosa | Several locations off the Japanese coast | [23] | |
| 10-Isocyano-4-cadinene (64) | 231 | C16H25N | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] |
| - | - | A. cavernosa | Several locations off the Japanese coast | [23] | |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [9] | |
| 10-Formamido-4-cadinene (65) | 249 | C16H27NO | A. cavernosa | Several locations off the Japanese coast | [23] |
| - | - | A. cavernosa | Xidao Island, Hainan, China | [22] | |
| (+)-α-Muurolene (66) | 204 | C15H24 | A. cavernosa | Hachijo-jima Island, Japan | [11] |
| T-cadinol (67) | 222 | C15H26O | A. cavernosa | Several locations off the Japanese coast | [23] |
| (+)-Maninsigin D (68) | 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [14] |
| 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [14] | |
| (-)-Maninsigin D (69) | 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [14] |
| (+)-Ximaocavernosin Q (70) | 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [14] |
| (-)-Ximaocavernosin Q (71) | 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [14] |
| Cadalene (72) | 198 | C15H18 | A. cavernosa | Coast of Ximao Island, Hainan, China | [14] |
| trans-4,5-Dihydroxycorocalane (73) | 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [14] |
| Axane-type sesquiterpenes | |||||
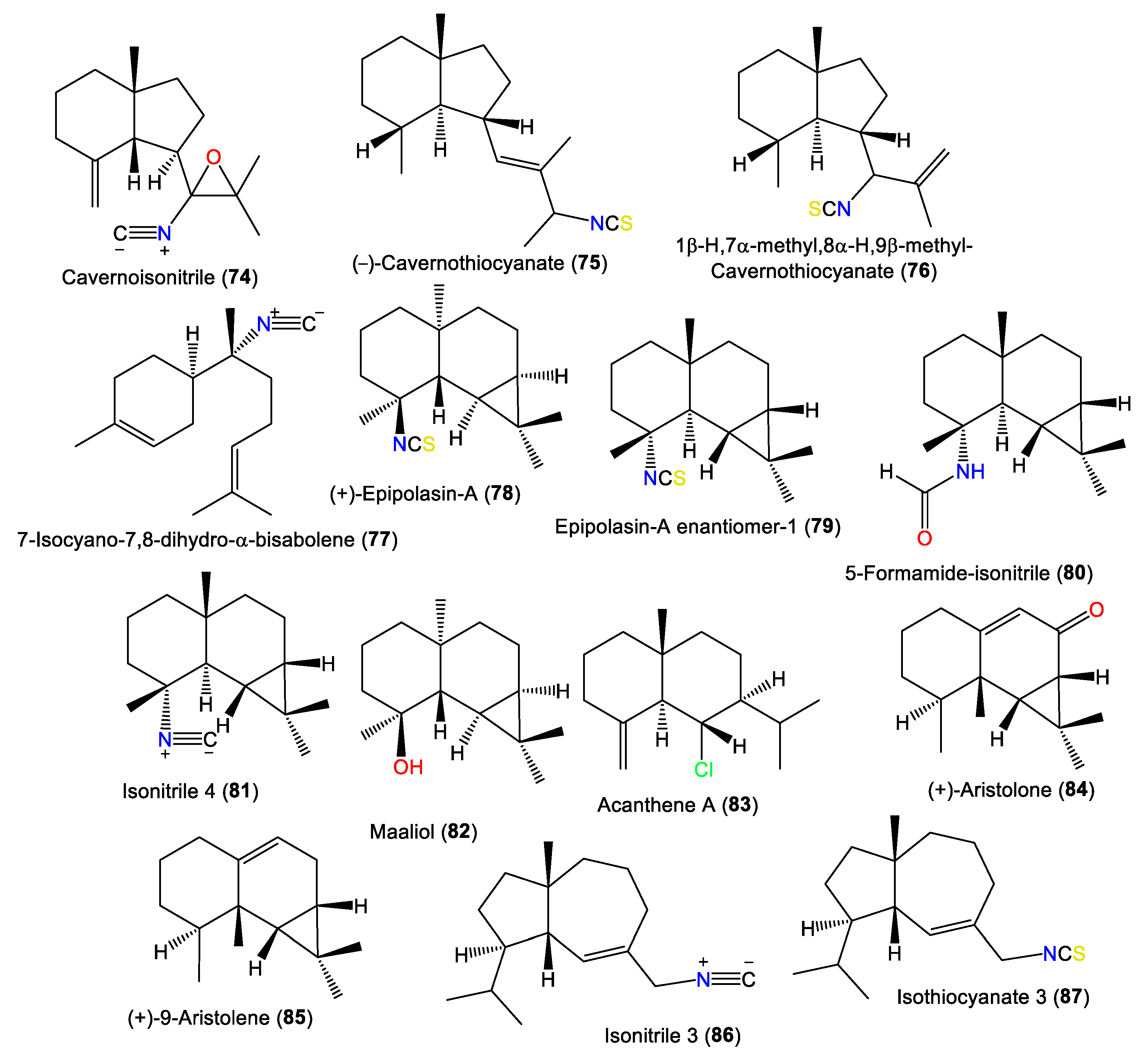
| Cavernoisonitrile (74) | 245 | C16H23NO | A. cavernosa | Hachijo-Jima Island, Japan | [3] |
| (-)-Cavernothiocyanate (75) | 263 | C16H25NS | A. cavernosa | Hachijo-Jima Island, Japan | [3] |
| - | - | A. cavernosa | Hachijo-jima Island, Japan | [11] | |
| 1β-H,7α-methyl,8α-H,9β-methyl-Cavernothiocyanate (76) | 263 | C16H25NS | A. cavernosa | Hachijo-jima Island, Japan | [11] |
| Bisabolene-type sesquiterpenes | |||||
| 7-Isocyano-7,8-dihydro-α-bisabolene (77) | 231 | C16H25N | A. cavernosa | Hachijo-Jima Island, Japan | [3] |
| Epimaaliane-type sesquiterpenes | |||||
| (+)-Epipolasin-A (78) | 263 | C16H25NS | A. pulcherrima | Weed Reef, Darwin, Australia | [6] |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [2] | |
| Epipolasin-A enantiomer-1 (79) | 263 | C16H25NS | A. pulcherrima | Weed Reef, Darwin, Australia | [6] |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [12] | |
| 5-Formamide-isonitrile (80) | 249 | C16H27NO | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [20] |
| Isonitrile 4 (81) | 231 | C16H25N | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [20] |
| Maaliol (82) | 222 | C15H26O | A. pulcherrima | Weed Reef, Darwin, Australia | [6] |
| Acanthene A (83) | 240 | C15H25Cl | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [20] |
| Gurjunene | |||||
| (+)-Aristolone (84) | 218 | C15H22O | A. cavernosa | Coast of Ximao Island, Hainan, China | [14] |
| - | - | A. cavernosa | South China Sea | [24] | |
| (+)-9-Aristolene (85) | 204 | C15H24 | A. cavernosa | Hachijo-jima Island, Japan | [11] |
| Isonitrile 3 (86) | 231 | C16H25N | A. acuta | Bay of Naples, southern Italy | [10] |
| Isothiocyanate 3 (87) | 263 | C16H25NS | A. acuta | Bay of Naples, southern Italy | [10] |
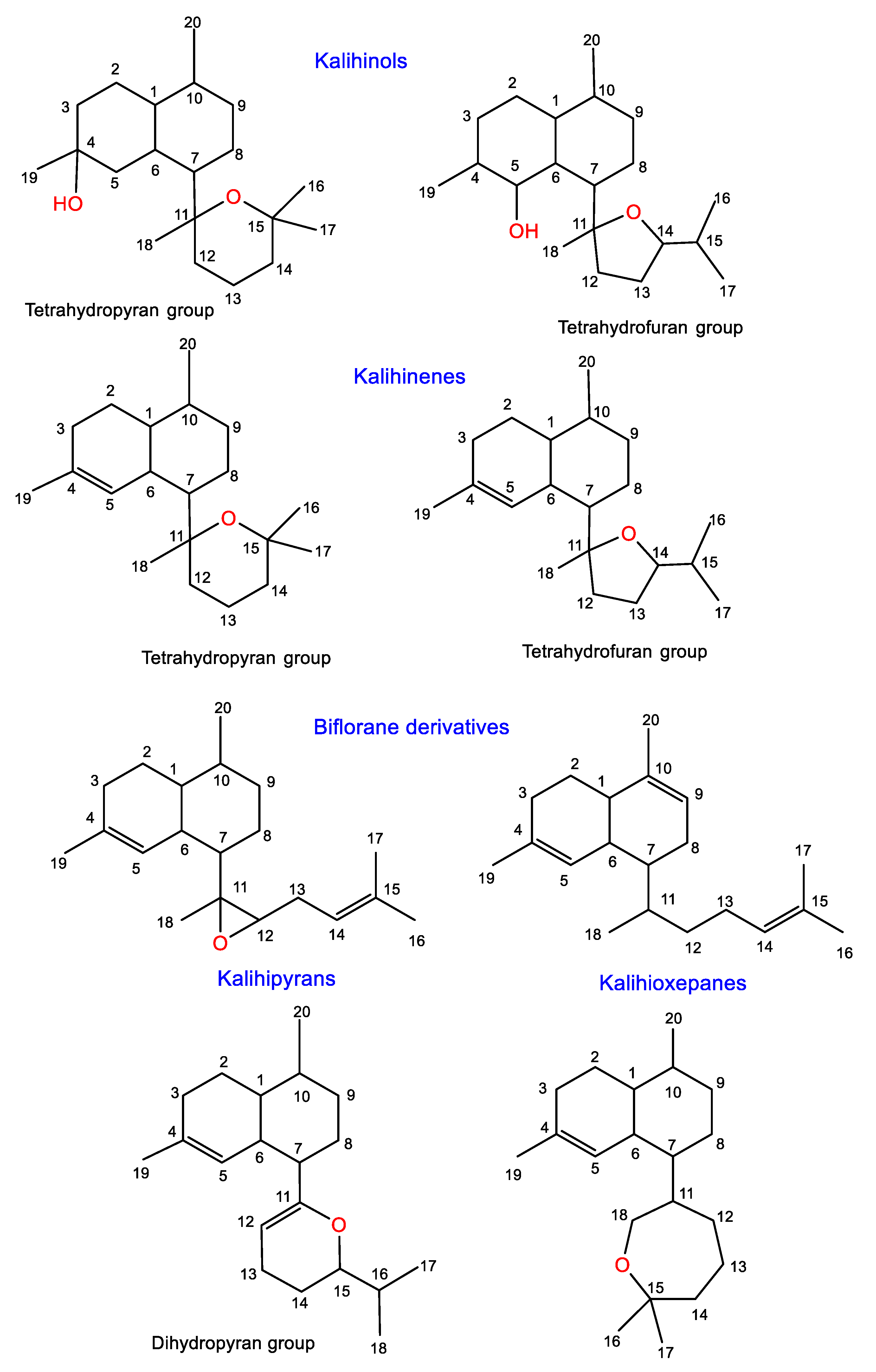
1.1. Aromadendrane-Type Sesquiterpenes
1.2. Spiroaxane-Type Sesquiterpenes
1.3. Eudesmane-Type Sesquiterpenes
1.4. Cadinene-Type Sesquiterpenes
1.5. Other Sesquiterpenes
2. Diterpenoids
| Compound Name | Mol. Wt. | Mol. Formula | Species | Sampling Locations | Ref. |
|---|---|---|---|---|---|
| Kalihinols | |||||
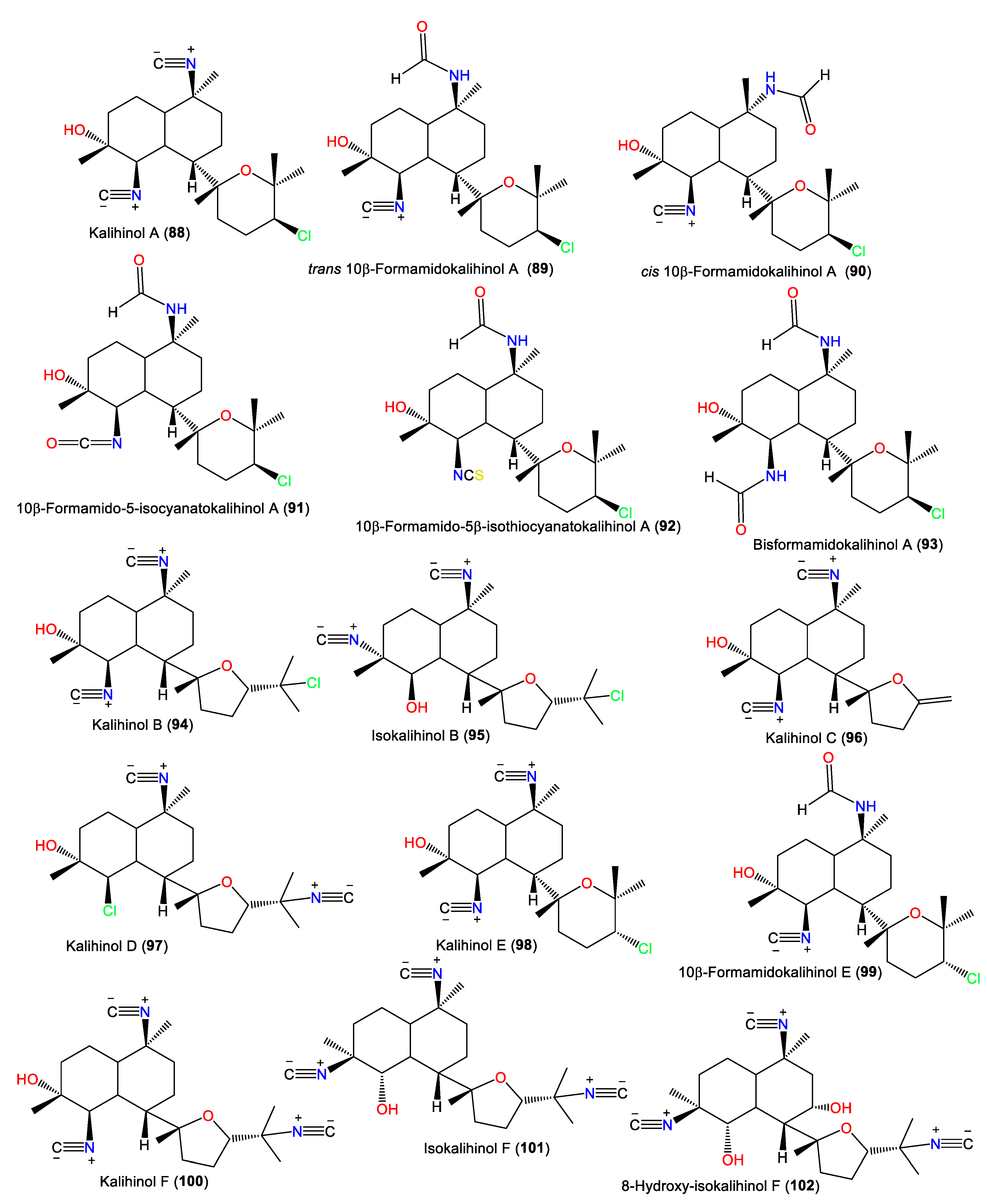
| Kalihinol A (88) | 392 | C22H33ClN2O2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [29] |
| - | - | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [30] | |
| - | - | A. caruenosa | Fiji, South Pacific Ocean | [31] | |
| - | - | A. klethra | Kuchinoerabu Island of the Satsunan Archipelago, Japan | [27] | |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [28] | |
| - | - | A. cavernosa | Yakushima Island, southwest of Tokyo | [33][34] | |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japa | [35] | |
| - | - | A. cavernosa | Dibud, Philippines | [36] | |
| - | - | A. cavernosa | Shallow water reef in Sanya Bay, Hainan Island, China | [37] | |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [12] | |
| - | - | Acanthella sp. | Yalong Bay, Hainan, China | [13] | |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [38] | |
| - | - | A. cavernosa | South China Sea | [24] | |
| trans 10β-Formamidokalihinol A (89) | 410 | C22H35ClN2O3 | A. cavernosa | Hachijo-jima Island, Japan | [11] |
| - | - | A. cavernosa | Shallow water reef in Sanya Bay, Hainan Island, China | [37] | |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [38] | |
| cis 10β-Formamidokalihinol A (90) | 410 | C22H35ClN2O3 | A. cavernosa | Shallow water reef in Sanya Bay, Hainan Island, China | [37] |
| 10β-Formamido-5-isocyanatokalihinol A (91) | 426 | C22H35ClN2O4 | A. cavernosa | Hachijo-jima Island, Japan | [11] |
| - | - | A. cavernosa | Xidao Island, Hainan, China | [22] | |
| 10β-Formamido-5β-isothiocyanatokalihinol A (92) | 442 | C22H35ClN2O3S | A. cavernosa | Hachijo-jima Island, Japan | [11] |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [38] | |
| - | - | A. cavernosa | Xidao Island, Hainan, China | [22] | |
| Bisformamidokalihinol A (93) | 428 | C22H37ClN2O4 | A. cavernosa | Xidao Island, Hainan, China | [22] |
| Kalihinol B (94) | 392 | C22H33ClN2O2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [30] |
| - | - | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [20] | |
| - | - | A. klethra | Kuchinoerabu Island of the Satsunan Archipelago, Japan | [27] | |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| Isokalihinol B (95) | 392 | C22H33ClN2O2 | A. klethra | Kuchinoerabu Island of the Satsunan Archipelago, Japan | [27] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| - | - | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [39] | |
| Kalihinol C (96) | 328 | C20H28N2O2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [30] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| Kalihinol D (97) | 392 | C22H33ClN2O2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [30] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| - | - | Acanthella sp. | Yalong Bay, Hainan, China | [13] | |
| Kalihinol E (98) | 392 | C22H35ClN2O3 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [30] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| - | - | A. cavernosa | Hachijo-jima Island, Japan | [11] | |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [38] | |
| - | - | A. cavernosa | South China Sea | [24] | |
| 10β-Formamidokalihinol E (99) | 410 | C22H33ClN2O2 | A. cavernosa | Hachijo-jima Island, Japan | [11] |
| Kalihinol F (100) | 383 | C23H33N3O2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [30] |
| - | - | A. caruenosa | Fiji, South Pacific Ocean | [31] | |
| - | - | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [40] | |
| - | - | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [20] | |
| - | - | A. klethra | Kuchinoerabu Island of the Satsunan Archipelago, Japan | [27] | |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| - | - | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [39] | |
| - | - | Acanthella sp. | Coast of Cape Sada, Ehime Prefecture, Japan | [41] | |
| - | - | A. cavernosa | Dibud, Philippines | [36] | |
| Isokalihinol F (101) | 383 | C23H33N3O2 | A. cavernosa | Fiji, South Pacific Ocean | [31] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| - | - | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [39] | |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [28] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] | |
| 8-Hydroxy-isokalihinol F (102) | 399 | C23H33N3O3 | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] |
| 10-epi-Isokalihinol F (103) | 383 | C23H33N3O2 | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [39] |
| trans 10-Formamido-kalihinol F (104) | 401 | C23H35N3O3 | A. cavernosa | Dibud, Philippines | [36] |
| cis 10-Formamido-kalihinol F (105) | 401 | C23H35N3O3 | A. cavernosa | Dibud, Philippines | [36] |
| trans 15-Formamido-kalihinol F (106) | 401 | C23H35N3O3 | A. cavernosa | Dibud, Philippines | [36] |
| cis 15-Formamido-kalihinol F (107) | 401 | C23H35N3O3 | A. cavernosa | Dibud, Philippines | [36] |
| Kalihinol G (108) | 415 | C23H33N3O2S | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [30] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [35] | |
| - | - | A. cavernosa | Dibud, Philippines | [36] | |
| 10-Isothiocyanatokalihinol G (109) | 447 | C23H33N3O2S2 | A. cavernosa | Xisha Islets, South China Sea | [38] |
| 5,10-bis-Isothiocyanatokalihinol G (110) | 479 | C23H33N3O2S3 | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [35] |
| Kalihinol H (111) | 415 | C23H33N3O2S | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [30] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [35] | |
| Isokalihinol H (112) | 415 | C23H33N3O2S | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [39] |
| 10-epi-Isokalihinol H (113) | 415 | C23H33N3O2S | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [39] |
| Kalihinol I (114) | 456 | C22H33ClN2O2S2 | A. cavernosa | Thailand | [15] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [35] | |
| 10-epi-Kalihinol I (115) | 456 | C22H33ClN2O2S2 | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [35] |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [38] | |
| - | - | A. cavernosa | South China Sea | [24] | |
| Kalihinol J (116) | 442 | C22H35ClN2O3S | A. cavernosa | Thailand | [15] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| - | - | A. cavernosa | Dibud, Philippines | [36] | |
| Kalihinol M (117) | 442 | C22H35ClN2O3S | A. cavernosa | Xisha Islets, South China Sea | [38] |
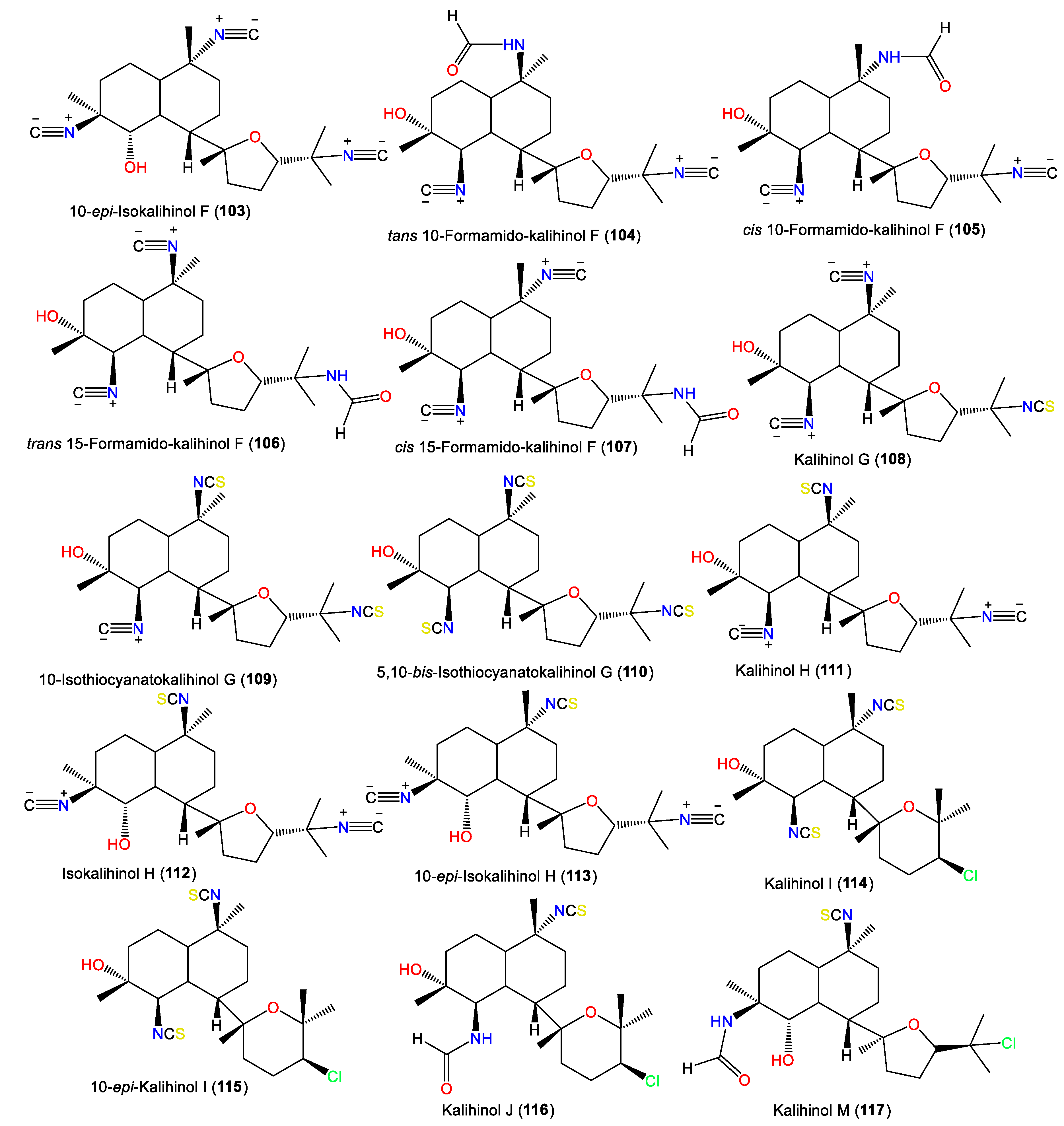
| Kalihinol N (118) | 442 | C22H35ClN2O3S | A. cavernosa | Xisha Islets, South China Sea | [38] |
| Kalihinol O (119) | 424 | C22H33ClN2O2S | A. cavernosa | Xisha Islets, South China Sea | [38] |
| Kalihinol P (120) | 424 | C22H33ClN2O2S | A. cavernosa | Xisha Islets, South China Sea | [38] |
| Kalihinol Q (121) | 424 | C22H33ClN2O2S | A. cavernosa | Xisha Islets, South China Sea | [38] |
| Kalihinol R (122) | 456 | C22H33ClN2O2S2 | A. cavernosa | Xisha Islets, South China Sea | [38] |
| Kalihinol S (123) | 410 | C22H35ClN2O3 | A. cavernosa | Xisha Islets, South China Sea | [38] |
| Kalihinol T (124) | 424 | C22H33ClN2O2S | A. cavernosa | Xisha Islets, South China Sea | [38] |
| Kalihinol X (125) | 424 | C22H33ClN2O2S | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [30] |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [31] | |
| - | - | A. cavernosa | Thailand | [15] | |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| - | - | A. cavernosa | Dibud, Philippines | [36] | |
| - | - | Acanthella sp. | Yalong Bay, Hainan, China | [13] | |
| 10-epi-Kalihinol X (126) | 424 | C22H33ClN2O2S | Acanthella sp. | Yalong Bay, Hainan, China | [13] |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [38] | |
| - | - | A. cavernosa | South China Sea | [24] | |
| Kalihinol Y (127) | 365 | C21H32ClNO2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [30] |
| - | - | Acanthella caruenosa | Fiji, South Pacific Ocean | [31] | |
| - | - | A. cavernosa | Thailand | [15] | |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [35] | |
| - | - | A. cavernosa | Dibud, Philippines | [36] | |
| Kalihinone Ya (128) | 367 | C20H30ClNO3 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [30] |
| Δ9-Kalihinol Y (129) | 365 | C21H32ClNO2 | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [35] |
| Kalihinol Z (130) | 392 | C22H33ClN2O2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [30] |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [31] | |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [32] | |
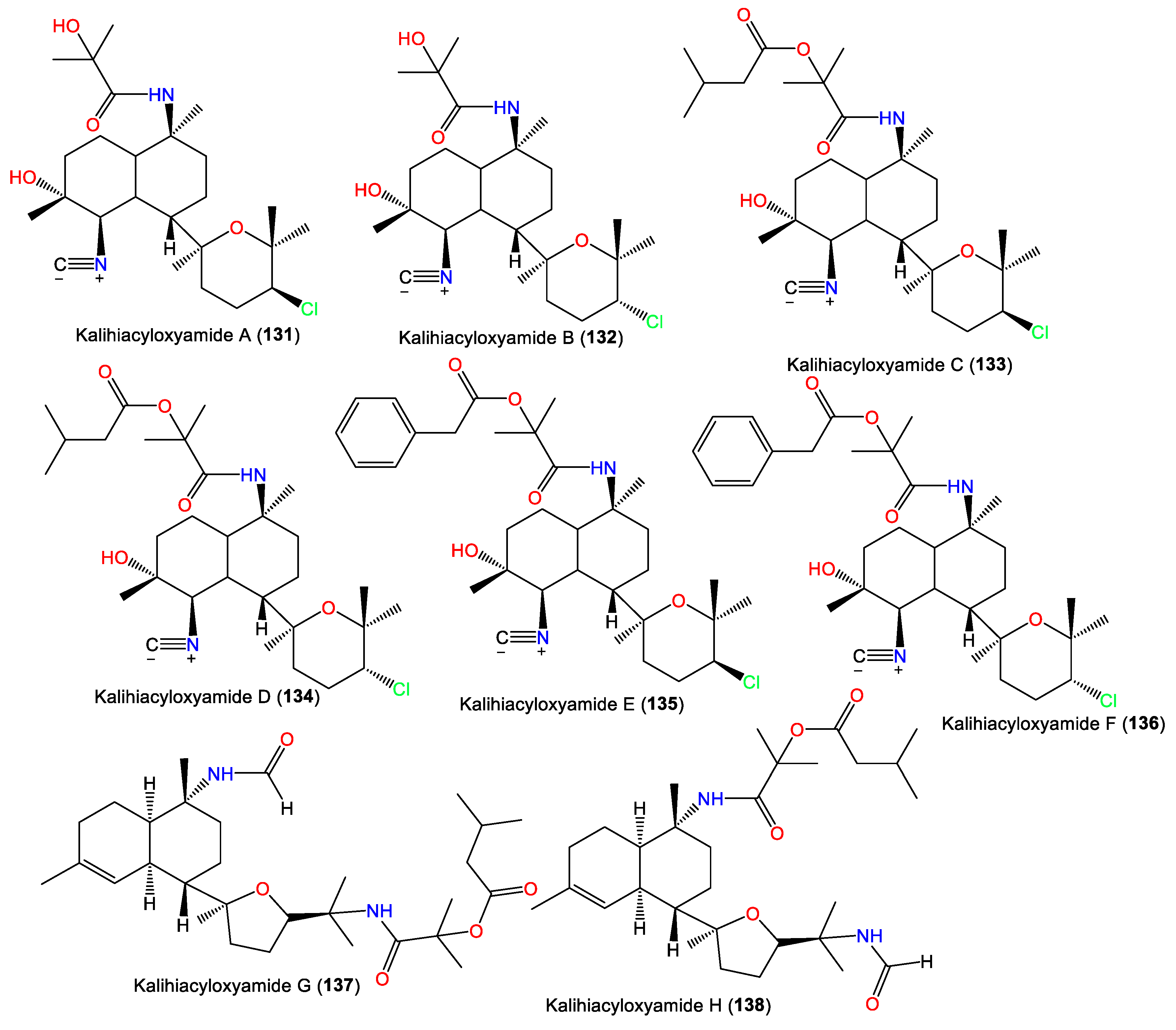
| Kalihiacyloxyamide A (131) | 468 | C25H41ClN2O4 | A. cavernosa | Xisha Island, South China Sea | [42] |
| Kalihiacyloxyamide B (132) | 468 | C25H41ClN2O4 | A. cavernosa | Xisha Island, South China Sea | [42] |
| Kalihiacyloxyamide C (133) | 552 | C30H49ClN2O5 | A. cavernosa | Xisha Island, South China Sea | [42] |
| Kalihiacyloxyamide D (134) | 552 | C30H49ClN2O5 | A. cavernosa | Xisha Island, South China Sea | [42] |
| Kalihiacyloxyamide E (135) | 586 | C33H47ClN2O5 | A. cavernosa | Xisha Island, South China Sea | [42] |
| Kalihiacyloxyamide F (136) | 586 | C33H47ClN2O5 | A. cavernosa | Xisha Island, South China Sea | [42] |
| Kalihiacyloxyamide G (137) | 518 | C30H50N2O5 | A. cavernosa | Xisha Island, South China Sea | [42] |
| Kalihiacyloxyamide H (138) | 518 | C30H50N2O5 | A. cavernosa | Xisha Island, South China Sea | [42] |
| Kalihinene | |||||
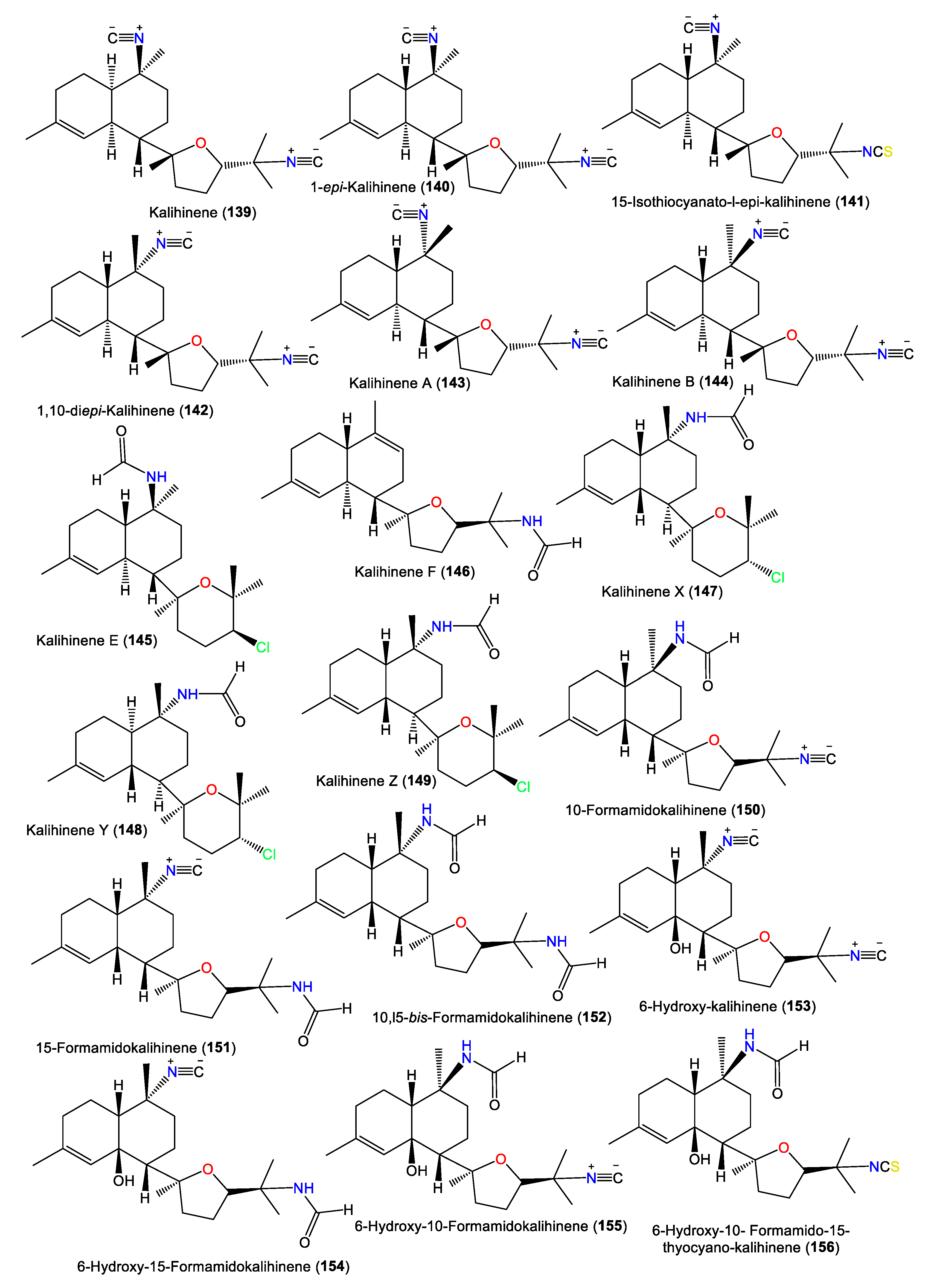
| Kalihinene (139) | 340 | C22H32N2O | A. klethra | Kuchinoerabu Island of the Satsunan Archipelago, Japan | [27] |
| - | - | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [39] | |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [28] | |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [35] | |
| - | - | A. cavernosa | Dibud, Philippines | [36] | |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [12] | |
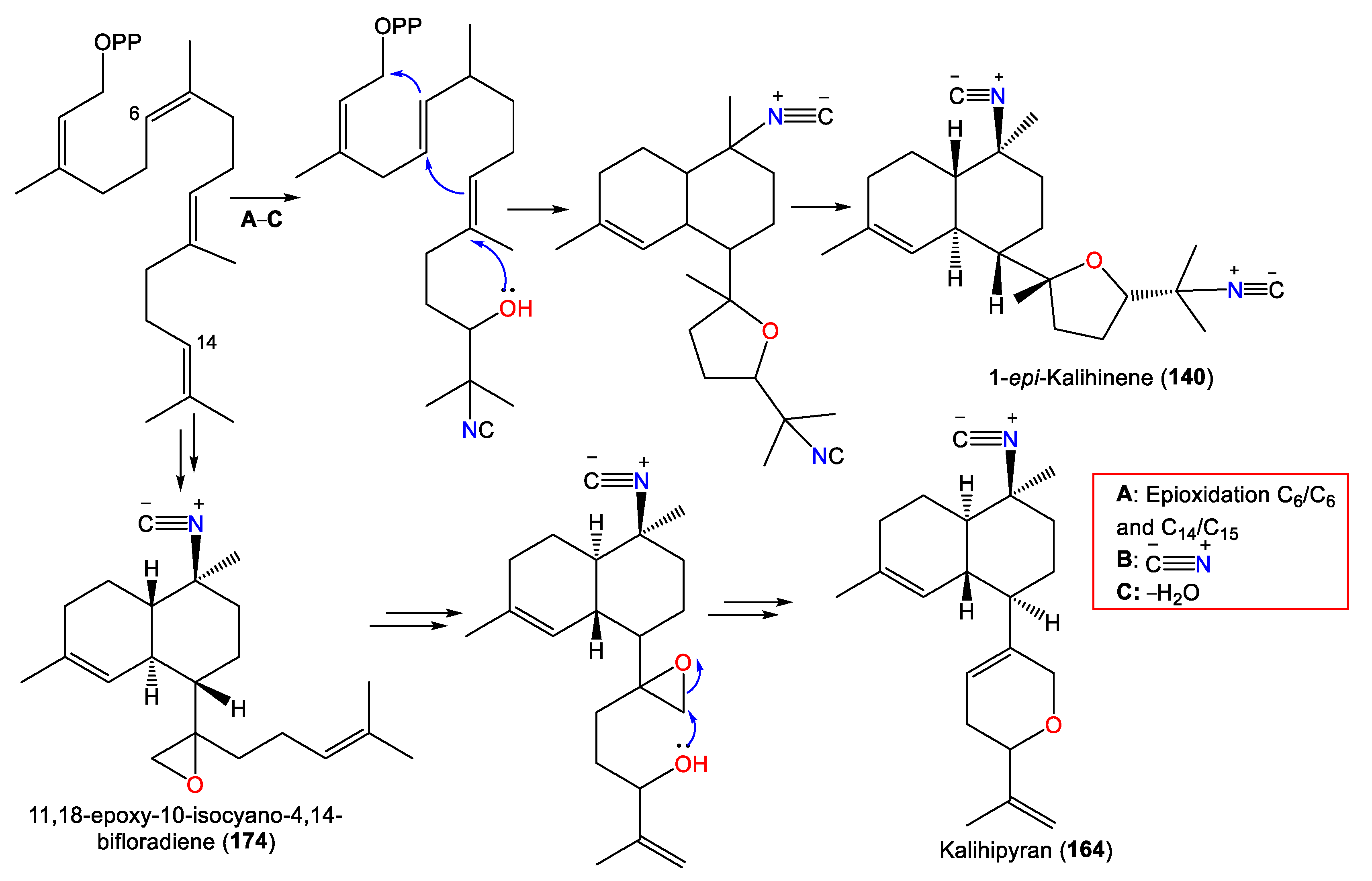
| 1-epi-Kalihinene (140) | 340 | C22H32N2O | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [39] |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] | |
| 15-Isothiocyanato-l-epi-kalihinene (141) | 372 | C22H32N2OS | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [39] |
| 1,10-diepi-Kalihinene (142) | 340 | C22H32N2O | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [39] |
| Kalihinene A (143) | 340 | C22H32N2O | A. cavernosa | Seychelles and Desnceufs Islands | [32] |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [43] | |
| Kalihinene B (144) | 340 | C22H32N2O | A. cavernosa | Seychelles and Desnceufs Islands | [32] |
| Kalihinene E (145) | 367 | C21H34ClNO2 | A. cavernosa | Xisha Islets, South China Sea | [43] |
| Kalihinene F (146) | 331 | C21H33NO2 | A. cavernosa | Xisha Islets, South China Sea | [43] |
| Kalihinene X (147) | 367 | C21H34ClNO2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [33][34] |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [43] | |
| Kalihinene Y (148) | 367 | C21H34ClNO2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [33][34] |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [43] | |
| Kalihinene Z (149) | 367 | C21H34ClNO2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [33][34] |
| 10-Formamidokalihinene (150) | 358 | C22H34N2O2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [33][34] |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [28] | |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [43] | |
| 15-Formamidokalihinene (151) | 358 | C22H34N2O2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [33] |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [28] | |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [43] | |
| 10,l5-bis-Formamidokalihinene (152) | 376 | C22H36N2O3 | A. cavernosa | Fiji, South Pacific Ocean | [28] |
| 6-Hydroxy-kalihinene (153) | 356 | C22H32N2O2 | A. cavernosa | Fiji, South Pacific Ocean | [28] |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [35] | |
| 6-Hydroxy-15-Formamidokalihinene (154) | 374 | C22H34N2O3 | A. cavernosa | Fiji, South Pacific Ocean | [28] |
| 6-Hydroxy-10-Formamidokalihinene (155) | 374 | C22H34N2O3 | A. cavernosa | Fiji, South Pacific Ocean | [28] |
| 6-Hydroxy-10-Formamido-15-thyocyano-kalihinene (156) | 406 | C22H34N2O3S | A. cavernosa | Fiji, South Pacific Ocean | [28] |
| Kalihioxepanes | |||||
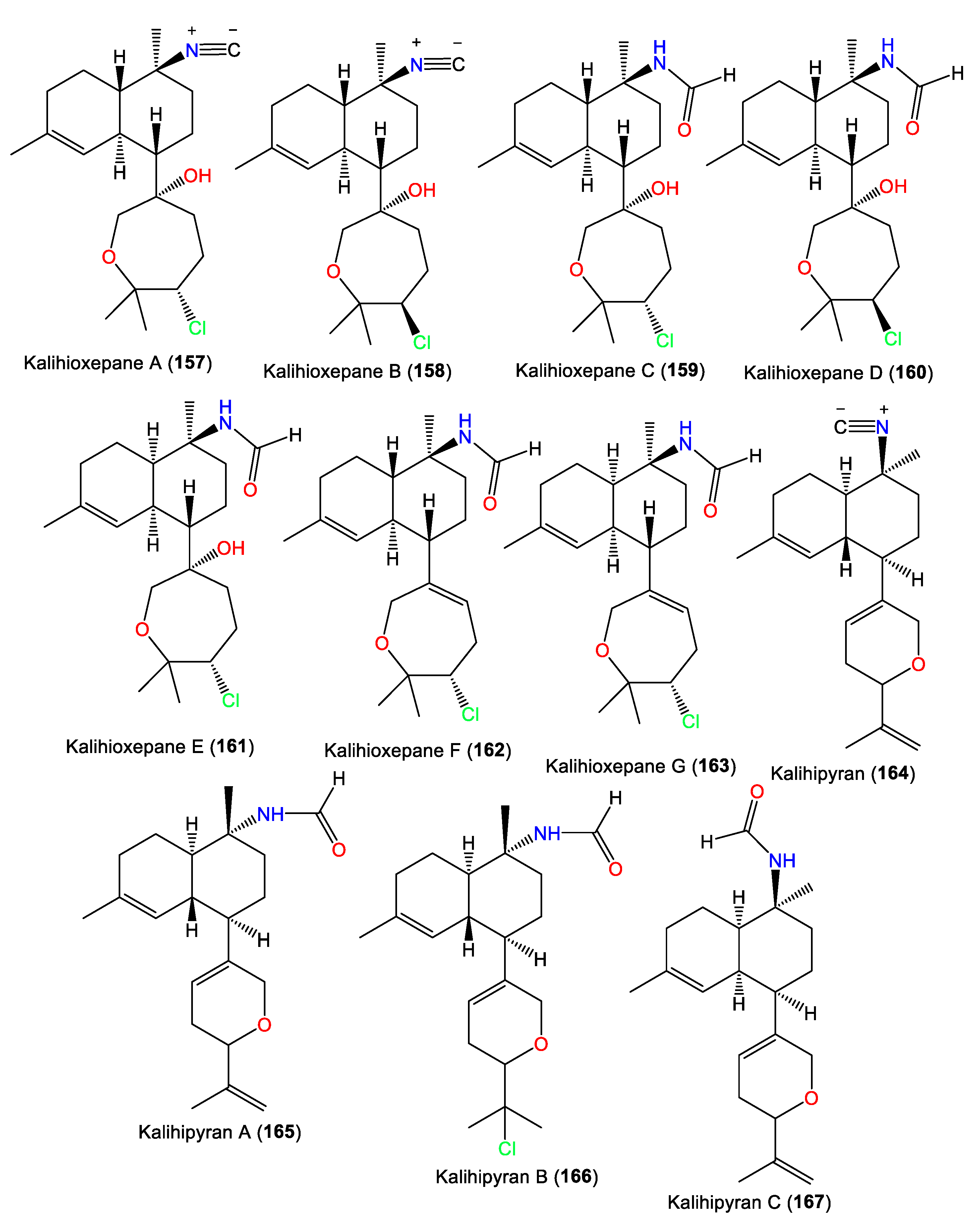
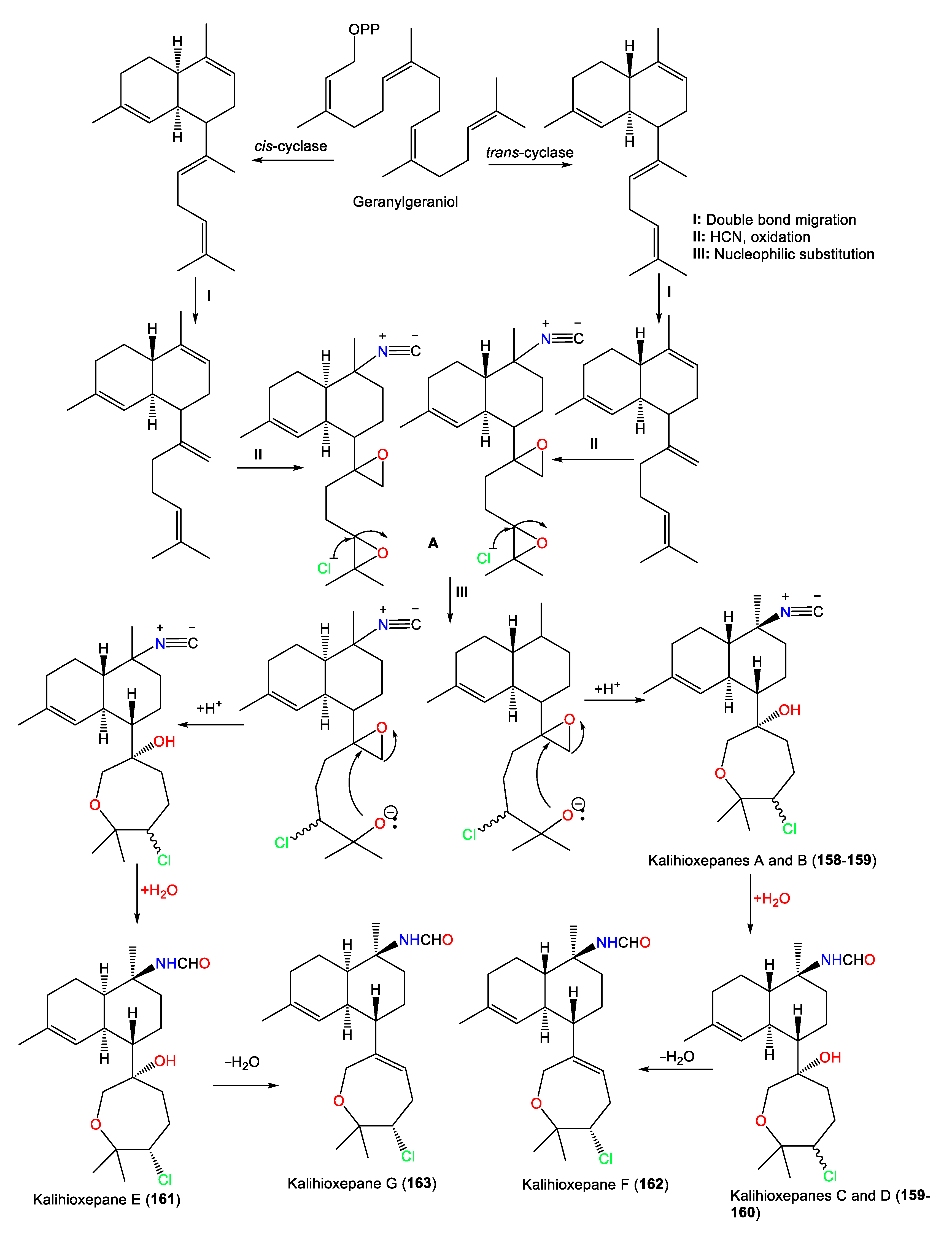
| Kalihioxepane A (157) | 365 | C21H32ClNO2 | A. cavernosa | Xisha Island, South China Sea | [44] |
| Kalihioxepane B (158) | 365 | C21H32ClNO2 | A. cavernosa | Xisha Island, South China Sea | [44] |
| Kalihioxepane C (159) | 383 | C21H34ClNO3 | A. cavernosa | Xisha Island, South China Sea | [44] |
| Kalihioxepane D (160) | 383 | C21H34ClNO3 | A. cavernosa | Xisha Island, South China Sea | [44] |
| Kalihioxepane E (161) | 383 | C21H34ClNO3 | A. cavernosa | Xisha Island, South China Sea | [44] |
| Kalihioxepane F (162) | 365 | C21H32ClNO2 | A. cavernosa | Xisha Island, South China Sea | [44] |
| Kalihioxepane G (163) | 365 | C21H32ClNO2 | A. cavernosa | Xisha Island, South China Sea | [44] |
| Kalihipyran (164) | 311 | C21H29NO | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [39] |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] | |
| Kalihipyrans | |||||
| Kalihipyran A (165) | 329 | C21H31NO2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [33] |
| Kalihipyran B (166) | 365 | C21H32ClNO2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [33] |
| Kalihipyran C (167) | 329 | C21H31NO2 | A. cavernosa | Xisha Islets, South China Sea | [43] |
| Biflorane diterpenes | |||||
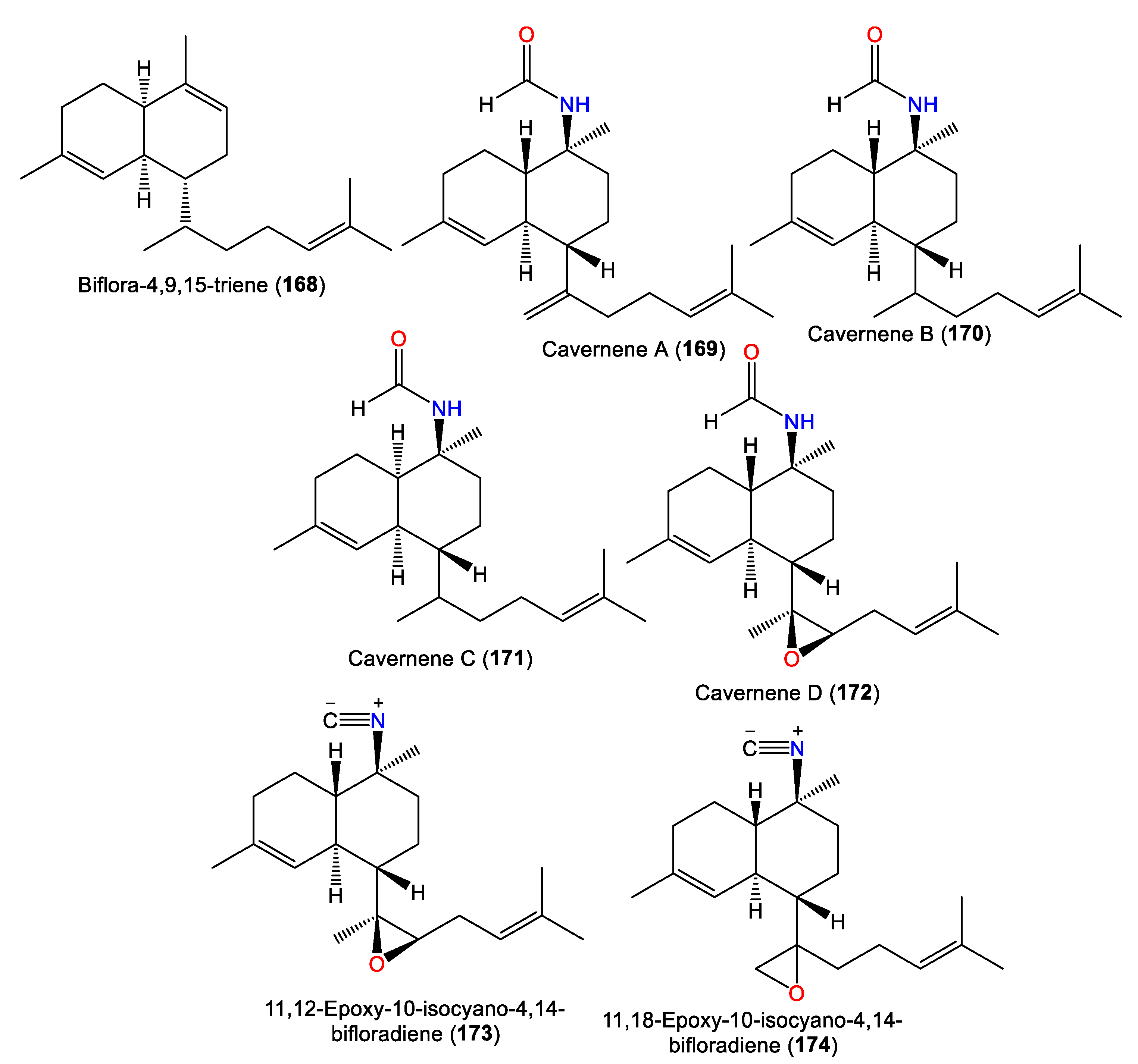
| Biflora-4,9,15-triene (168) | 272 | C20H32 | A. cavernosa | Yakushima Island, southwest of Tokyo | [33] |
| - | - | A. cavernosa | Hachijo-jima Island, Japan | [11] | |
| Cavernene A (169) | 315 | C21H33NO | A. cavernosa | Xisha Islets, South China Sea | [43] |
| Cavernene B (170) | 317 | C21H35NO | A. cavernosa | Xisha Islets, South China Sea | [43] |
| Cavernene C (171) | 317 | C21H35NO | A. cavernosa | Xisha Islets, South China Sea | [43] |
| Cavernene D (172) | 331 | C21H33NO2 | A. cavernosa | Xisha Islets, South China Sea | [43] |
| Isocyanobifloradiene epoxide A (173) 11,12-epoxy-10-isocyano-4,14-bifloradiene (173) |
313 | C21H31NO | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] |
| Isocyanobifloradiene epoxide B (174) 11,18-epoxy-10-isocyano-4,14-bifloradiene (174) |
313 | C21H31NO | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [18] |
2.1. Kalihinols
2.2. Kalihinenes
2.3. Kalihipyrans and Kalihioxepanes
2.4. Biflorane Diterpenes
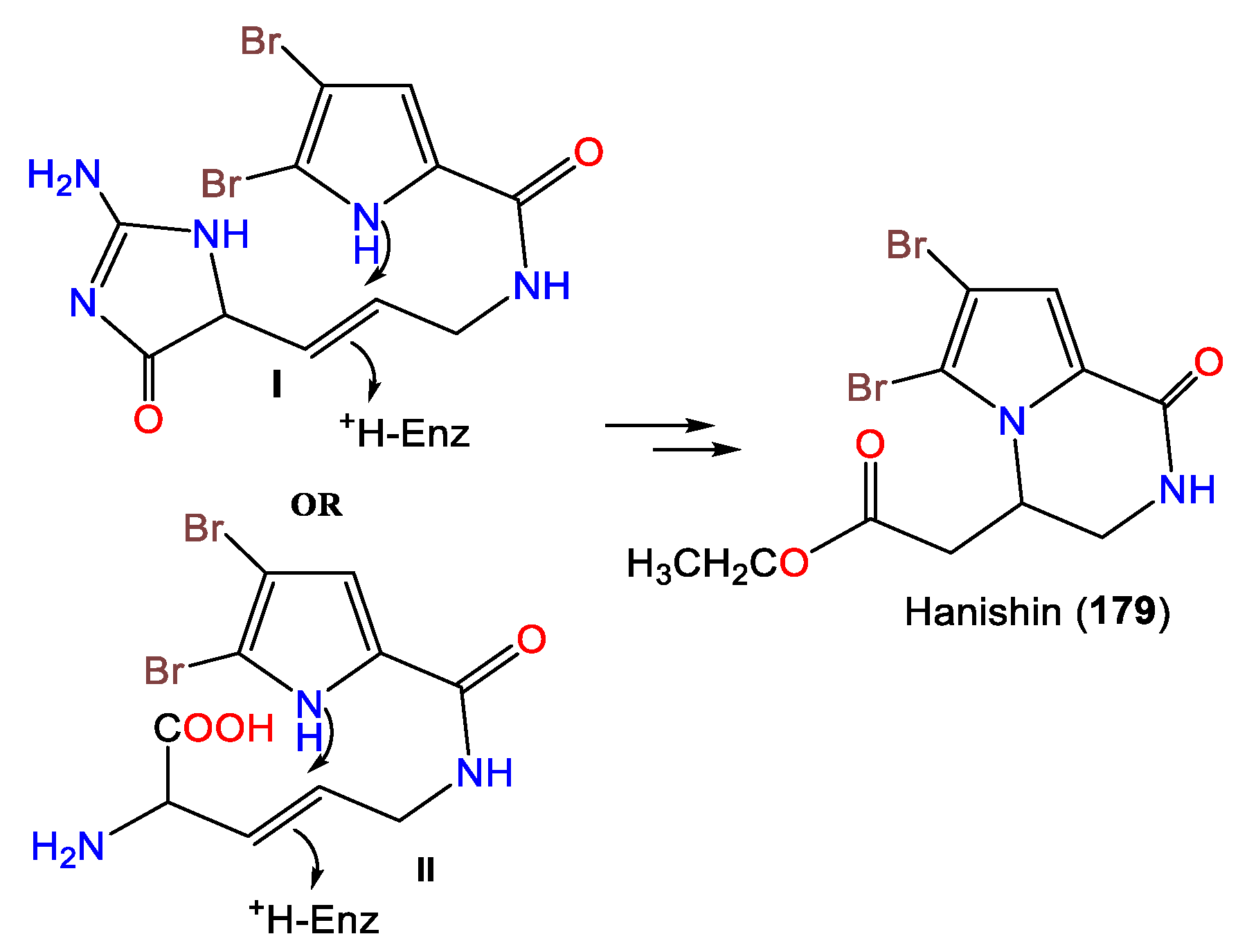
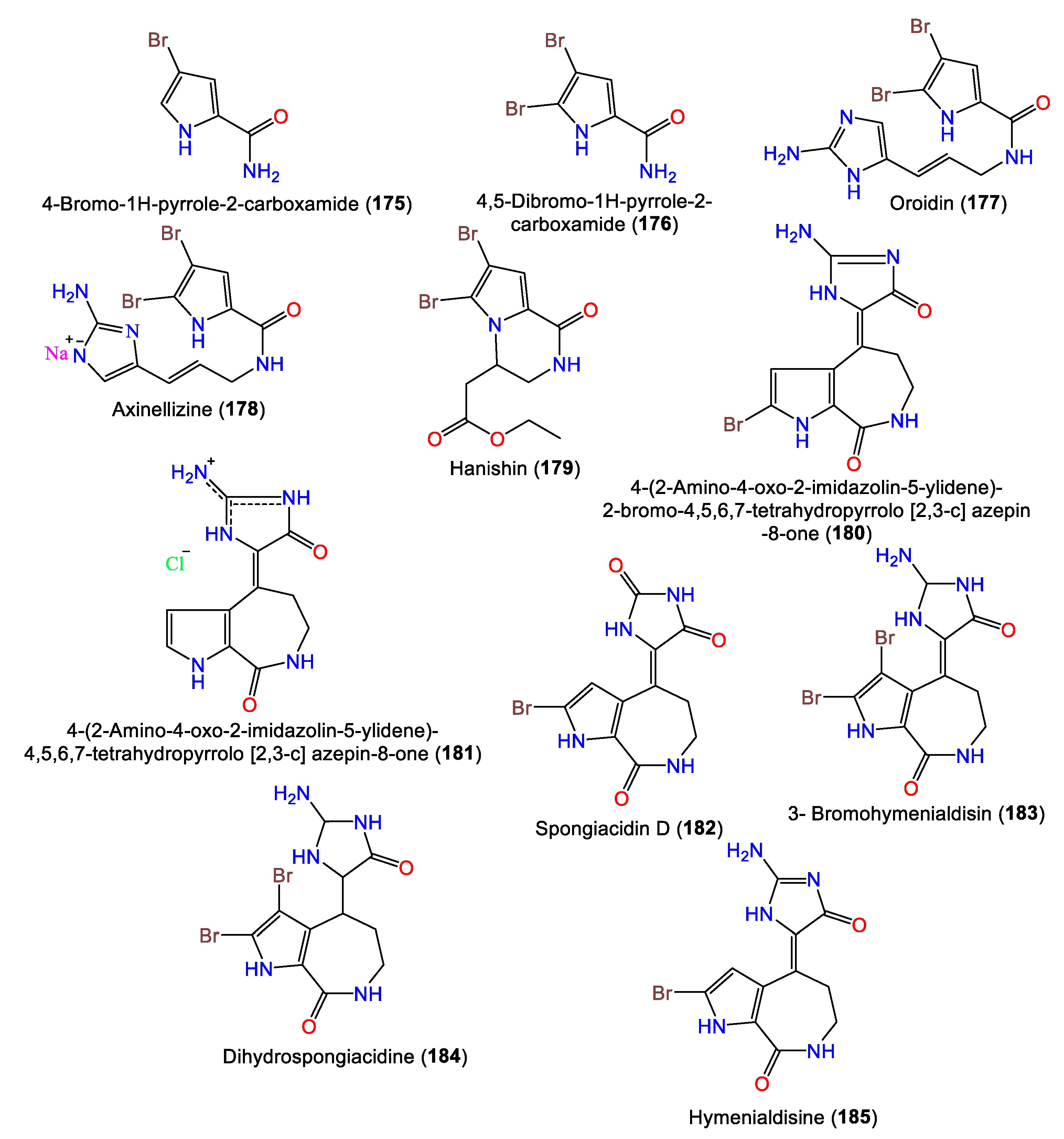
3. Alkaloids
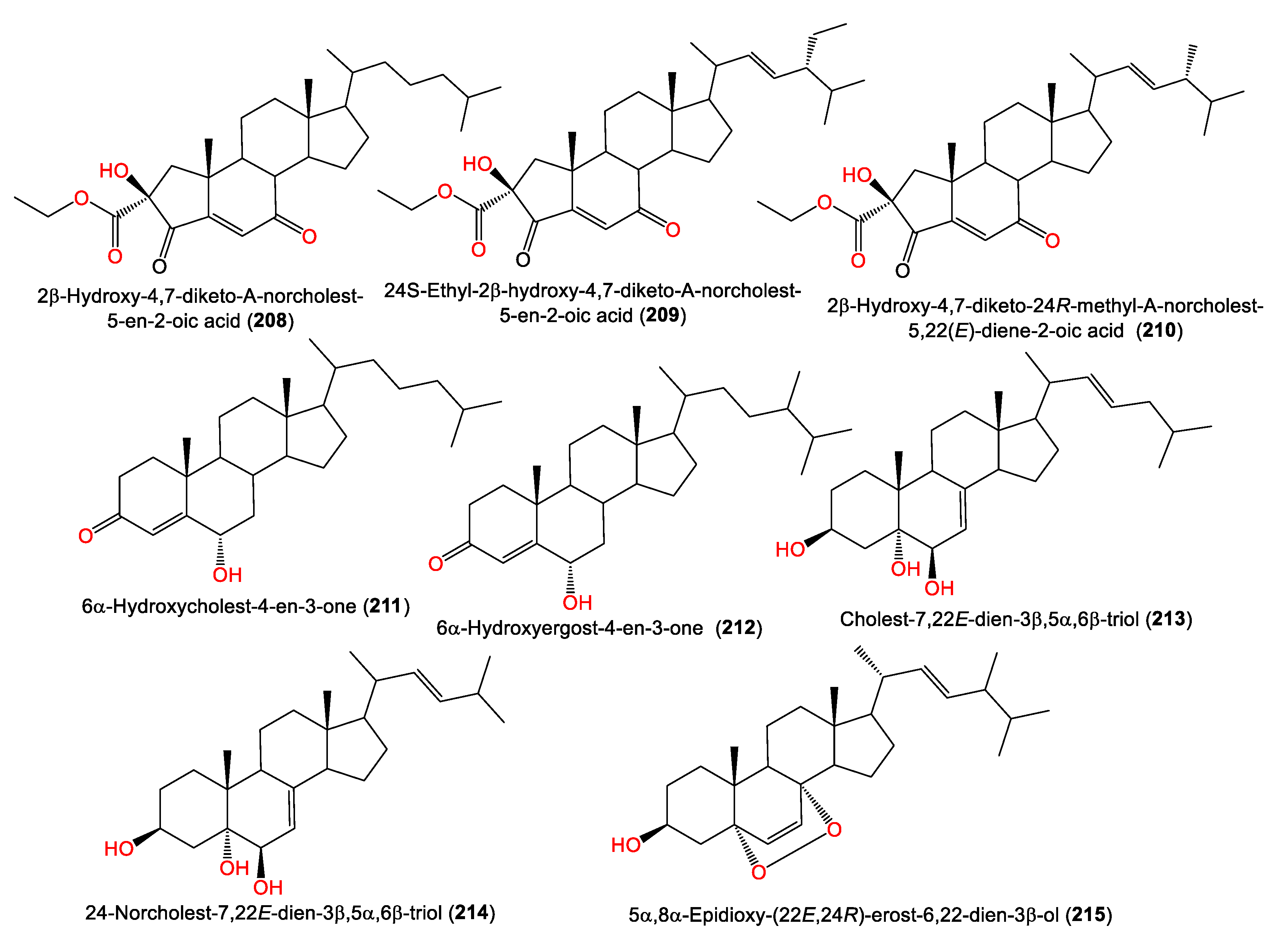
4. Steroid Compounds
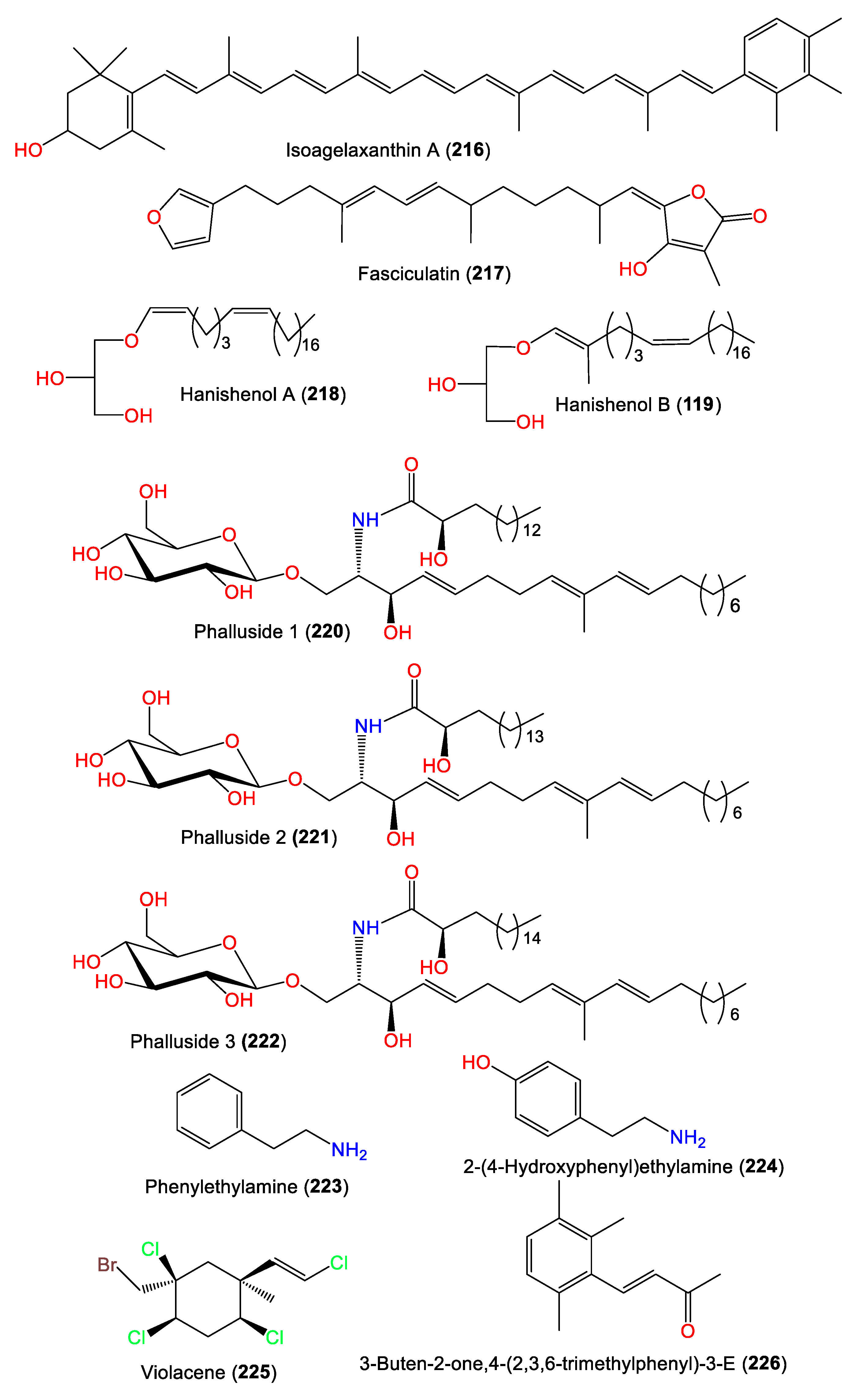
5. Other Metabolites
This entry is adapted from the peer-reviewed paper 10.3390/md21040257
References
- Minale, L.; Riccio, R.; Sodano, G. Acanthellin-1, an Unique Isonitrile Sesquiterpene from the Sponge Acanthella acuta. Tetrahedron 1974, 30, 1341–1343.
- Dumdei, E.J.; Flowers, A.E.; Garson, M.J.; Moore, C.J. The Biosynthesis of Sesquiterpene Isocyanides and Isothiocyanates in the Marine Sponge Acanthella cavernosa (Dendy); Evidence for Dietary Transfer to the Dorid Nudibranch Phyllidiella pustulosa. Comp. Biochem. Physiol. Part A Physiol. 1997, 118, 1385–1392.
- Fusetani, N.; Wolstenholme, H.J.; Shinoda, K.; Asai, N.; Matsunaga, S.; Onuki, H.; Hirota, H. Two Sesquiterpene Isocyanides and a Sesquiterpene Thiocyanate from the Marine Sponge Acanthella Cf. cavernosa and the Nudibranch Phyllidia ocellata. Tetrahedron Lett. 1992, 33, 6823–6826.
- Shen, S.; Zhang, Z.; Yao, L.; Wang, J.; Guo, Y.; Li, X. Nitrogenous Sesquiterpenoids from the South China Sea Nudibranch Hexabranchus sanguineus and its Possible Sponge-Prey Acanthella Cavernosa: Chiral Separation, Stereochemistry and Chemical Ecology. Chin. J. Chem. 2022, 40, 235–246.
- Braekman, J.C.; Daloze, D.; Deneubourg, F.; Huysecom, J.; Vandevyver, G. I-Isocyanoaromadendrane, A New Isonitrile Sesquiterpene from the Sponge Acanthella acuta. Bull. Sociétés Chim. Belg. 1987, 96, 539–543.
- Capon, R.J.; MacLeod, J.K. New Isothiocyanate Sesquiterpenes from the Australian Marine Sponge Acanthella pulcherrima. Aust. J. Chem. 1988, 41, 979–983.
- Braekman, J.C.; Daloze, D.; Moussiaux, B.; Stoller, C.; Deneubourg, F. Sponge Secondary Metabolites: New Results. Pure Appl. Chem. 1989, 61, 509–512.
- Jumaryatno, P.; Rands-Trevor, K.; Blanchfield, J.T.; Garson, M.J. Isocyanates in Marine Sponges: Axisocyanate-3, a New Sesquiterpene from Acanthella cavernosa. ARKIVOC 2007, vii, 157–166.
- Jumaryatno, P.; Stapleton, B.L.; Hooper, J.N.; Brecknell, D.J.; Blanchfield, J.T.; Garson, M.J. A Comparison of Sesquiterpene Scaffolds Across Different Populations of the Tropical Marine Sponge Acanthella cavernosa. J. Nat. Prod. 2007, 70, 1725–1730.
- Mayol, L.; Piccialli, V.; Sica, D. Nitrogenous Sesquiterpenes from the Marine Sponge Acanthella acuta: Three New Isocyanide-Isothiocyanate Pairs. Tetrahedron 1987, 43, 5381–5388.
- Hirota, H.; Tomono, Y.; Fusetani, N. Terpenoids with Antifouling Activity Against Barnacle Larvae from the Marine Sponge Acanthella cavernosa. Tetrahedron 1996, 52, 2359–2368.
- Yan, X.; Zhu, X.; Yu, J.; Jin, D.; Guo, Y.; Mollo, E.; Cimino, G. 3-Oxo-Axisonitrile-3, a New Sesquiterpene Isocyanide from the Chinese Marine Sponge Acanthella Sp. J. Asian Nat. Prod. Res. 2006, 8, 579–584.
- Sun, J.; Chen, K.; Yao, L.; Liu, H.; Guo, Y. A New Kalihinol Diterpene from the Hainan Sponge Acanthella Sp. Arch. Pharm. Res. 2009, 32, 1581–1584.
- Shen, S.; Yang, Q.; Zang, Y.; Li, J.; Liu, X.; Guo, Y. Anti-Inflammatory Aromadendrane-and Cadinane-Type Sesquiterpenoids from the South China Sea Sponge Acanthella cavernosa. Beilstein J. Org. Chem. 2022, 18, 916–925.
- Alvi, K.A.; Tenenbaum, L.; Crews, P. Anthelmintic Polyfunctional Nitrogen-Containing Terpenoids from Marine Sponges. J. Nat. Prod. 1991, 54, 71–78.
- Angerhofer, C.K.; Pezzuto, J.M.; König, G.M.; Wright, A.D.; Sticher, O. Antimalarial Activity of Sesquiterpenes from the Marine Sponge Acanthella klethra. J. Nat. Prod. 1992, 55, 1787–1789.
- König, G.M.; Wright, A.D.; Sticher, O.; Fronczek, F.R. Two New Sesquiterpene Isothiocyanates from the Marine Sponge Acanthella klethra. J. Nat. Prod. 1992, 55, 633–638.
- Clark, R.J.; Stapleton, B.L.; Garson, M.J. New Isocyano and Isothiocyanato Terpene Metabolites from the Tropical Marine Sponge Acanthella cavernosa. Tetrahedron 2000, 56, 3071–3076.
- Hammami, S.; Bergaoui, A.; Boughalleb, N.; Romdhane, A.; Khoja, I.; Kamel, M.B.H.; Mighri, Z. Antifungal Effects of Secondary Metabolites Isolated from Marine Organisms Collected from the Tunisian Coast. C. R. Chim. 2010, 13, 1397–1400.
- Burgoyne, D.L.; Dumdei, E.J.; Andersen, R.J. Acanthenes A to C: A Chloro, Isothiocyanate, Formamide Sesquiterpene Triad Isolated from the Northeastern Pacific Marine Sponge Acanthella Sp. and the Dorid Nudibranch Cadlina luteomarginata. Tetrahedron 1993, 49, 4503–4510.
- Ciminiello, P.; Magno, S.; Mayol, L.; Piccialli, V. Cis-Eudesmane Nitrogenous Metabolites from the Marine Sponges Axinella Cannabina and Acanthella acuta. J. Nat. Prod. 1987, 50, 217–220.
- Wu, Q.; Chen, W.; Li, S.; Ye, J.; Huan, X.; Gavagnin, M.; Yao, L.; Wang, H.; Miao, Z.; Li, X. Cytotoxic Nitrogenous Terpenoids from Two South China Sea Nudibranchs Phyllidiella pustulosa, Phyllidia Coelestis, and their Sponge-Prey Acanthella cavernosa. Mar. Drugs 2019, 17, 56.
- Nogata, Y.; Yoshimura, E.; Shinshima, K.; Kitano, Y.; Sakaguchi, I. Antifouling Substances Against Larvae of the Barnacle Balanus Amphitrite from the Marine Sponge, Acanthella cavernosa. Biofouling 2003, 19, 193–196.
- Fan, W.; Wang, X.; Cai, H.; Sun, L.; Yang, L.; Nie, S. Chemical Analysis of the South China Sea Spine Body Sponge Acanthella cavernosa. J. Pharm. Pract. 2016, 34, 138–141, 166.
- Ibrahim, S.R.; Fadil, S.A.; Fadil, H.A.; Hareeri, R.H.; Abdallah, H.M.; Mohamed, G.A. Ethnobotanical Uses, Phytochemical Composition, Biosynthesis, and Pharmacological Activities of Carpesium abrotanoides L. (Asteraceae). Plants 2022, 11, 1598.
- Ibrahim, S.R.M.; Mohamed, G.A.; Khedr, A.I.M.; Zayed, M.F.; El-Kholy, A.A.S. Genus Hylocereus: Beneficial Phytochemicals, Nutritional Importance, and Biological Relevance—A Review. J. Food Biochem. 2018, 42, e12491.
- Fusetani, N.; Yasumuro, K.; Kawai, H.; Natori, T.; Brinen, L.; Clardy, J. Kalihinene and Isokalihinol B, Cytotoxic Diterpene Isonitriles from the Marine Sponge Acanthella klethra. Tetrahedron Lett. 1990, 31, 3599–3602.
- Rodríguez, J.; Nieto, R.M.; Hunter, L.M.; Diaz, M.C.; Crews, P.; Lobkovsky, E.; Clardy, J. Variation among Known Kalihinol and New Kalihinene Diterpenes from the Sponge Acanthella cavernosa. Tetrahedron 1994, 50, 11079–11090.
- Chang, C.W.; Patra, A.; Roll, D.M.; Scheuer, P.J.; Matsumoto, G.K.; Clardy, J. Kalihinol-A, a Highly Functionalized Diisocyano Diterpenoid Antibiotic from a Sponge. J. Am. Chem. Soc. 1984, 106, 4644–4646.
- Chang, C.W.; Patra, A.; Baker, J.A.; Scheuer, P.J. Kalihinols, Multifunctional Diterpenoid Antibiotics from Marine Sponges Acanthella Spp. J. Am. Chem. Soc. 1987, 109, 6119–6123.
- Omar, S.; Albert, C.; Fanni, T.; Crews, P. Polyfunctional Diterpene Isonitriles from Marine Sponge Acanthella carvenosa. J. Org. Chem. 1988, 53, 5971–5972.
- Braekman, J.C.; Daloze, D.; Gregoire, F.; Popov, S.; van Soest, R. Two New Kalihinenes from the Marine Sponge Acanthella cavernosa. Bull. Soc. Chim. Belg. 1994, 103, 187–191.
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. New Antifouling Kalihipyrans from the Marine Sponge Acanthella vavernosa. J. Nat. Prod. 1996, 59, 1081–1083.
- Okino, T.; Yoshimura, E.; Hirota, H.; Fusetani, N. Antifouling Kalihinenes from the Marine Sponge Acanthella cavernosa. Tetrahedron Lett. 1995, 36, 8637–8640.
- Miyaoka, H.; Shimomura, M.; Kimura, H.; Yamada, Y.; Kim, H.; Yusuke, W. Antimalarial Activity of Kalihinol A and New Relative Diterpenoids from the Okinawan Sponge, Acanthella Sp. Tetrahedron 1998, 54, 13467–13474.
- Bugni, T.S.; Singh, M.P.; Chen, L.; Arias, D.A.; Harper, M.K.; Greenstein, M.; Maiese, W.M.; Concepción, G.P.; Mangalindan, G.C.; Ireland, C.M. Kalihinols from Two Acanthella cavernosa Sponges: Inhibitors of Bacterial Folate Biosynthesis. Tetrahedron 2004, 60, 6981–6988.
- Yang, L.H.; Lee, O.O.; Jin, T.; Li, X.C.; Qian, P.Y. Antifouling Properties of 10β-Formamidokalihinol-A and Kalihinol A Isolated from the Marine Sponge Acanthella cavernosa. Biofouling 2006, 22, 23–32.
- Xu, Y.; Li, N.; Jiao, W.; Wang, R.; Peng, Y.; Qi, S.; Song, S.; Chen, W.; Lin, H. Antifouling and Cytotoxic Constituents from the South China Sea Sponge Acanthella cavernosa. Tetrahedron 2012, 68, 2876–2883.
- Trimurtulu, G.; Faulkner, D.J. Six New Diterpene Isonitriles from the Sponge Acanthella cavernosa. J. Nat. Prod. 1994, 57, 501–506.
- Karuso, P.; Scheuer, P.J. Biosynthesis of Isocyanoterpenes in Sponges. J. Org. Chem. 1989, 54, 2092–2095.
- Ohta, E.; Ohta, S.; Hongo, T.; Hamaguchi, Y.; Andoh, T.; Shioda, M.; Ikegami, S. Inhibition of Chromosome Separation in Fertilized Starfish Eggs by Kalihinol F, a Topoisomerase I Inhibitor obtained from a Marine Sponge. Biosci. Biotechnol. Biochem. 2003, 67, 2365–2372.
- Wang, Z.; Li, Y.; Han, X.; Zhang, D.; Hou, H.; Xiao, L.; Li, G. Kalihiacyloxyamides AH, A-Acyloxy Amide Substituted Kalihinane Diterpenes Isolated from the Sponge Acanthella cavernosa Collected in the South China Sea. Phytochemistry 2023, 206, 113512.
- Xu, Y.; Lang, J.; Jiao, W.; Wang, R.; Peng, Y.; Song, S.; Zhang, B.; Lin, H. Formamido-Diterpenes from the South China Sea Sponge Acanthella cavernosa. Mar. Drugs 2012, 10, 1445–1458.
- Wang, Z.; Han, X.; Liu, G.; Zhang, D.; Hou, H.; Xiao, L.; de Voogd, N.J.; Tang, X.; Li, P.; Li, G. Kalihioxepanes A—G: Seven Kalihinene Diterpenoids from Marine Sponge Acanthella cavernosa Collected Off the South China Sea. Chin. J. Chem. 2022, 40, 1785–1792.
- Shimomura, M.; Miyaoka, H.; Yamada, Y. Absolute Configuration of Marine Diterpenoid Kalihinol A. Tetrahedron Lett. 1999, 40, 8015–8017.
- Mancini, I.; Guella, G.; Amade, P.; Roussakis, C.; Pietra, F. Hanishin, a Semiracemic, Bioactive C9 Alkaloid of the Axinellid Sponge Acanthella ccarteri from the Hanish Islands. A Shunt Metabolite? Tetrahedron Lett. 1997, 38, 6271–6274.
- Mattia, C.A.; Mazzarella, L.; Puliti, R. 4-(2-Amino-4-Oxo-2-Imidazolin-5-Ylidene)-2-Bromo-4, 5, 6, 7-Tetrahydropyrrolo Azepin-8-One Methanol Solvate: A New Bromo Compound from the Sponge Acanthella aurantiaca. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 1982, 38, 2513–2515.
- Cimino, G.; De Rosa, S.; De Stefano, S.; Mazzarella, L.; Puliti, R.; Sodano, G. Isolation and X-Ray Crystal Structure of a Novel Bromo-Compound from Two Marine Sponges. Tetrahedron Lett. 1982, 23, 767–768.
- Macabeo, A.P.G.; Guce, F.d. Bromopyrrole-Imidazole Alkaloids from Acanthella carteri Dendy (Axinellidae). Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 720–723.
- qing Feng, D.; Qiu, Y.; Wang, W.; Wang, X.; gang Ouyang, P.; huan Ke, C. Antifouling Activities of Hymenialdisine and Debromohymenialdisine from the Sponge Axinella Sp. Int. Biodeterior. Biodegrad. 2013, 85, 359–364.
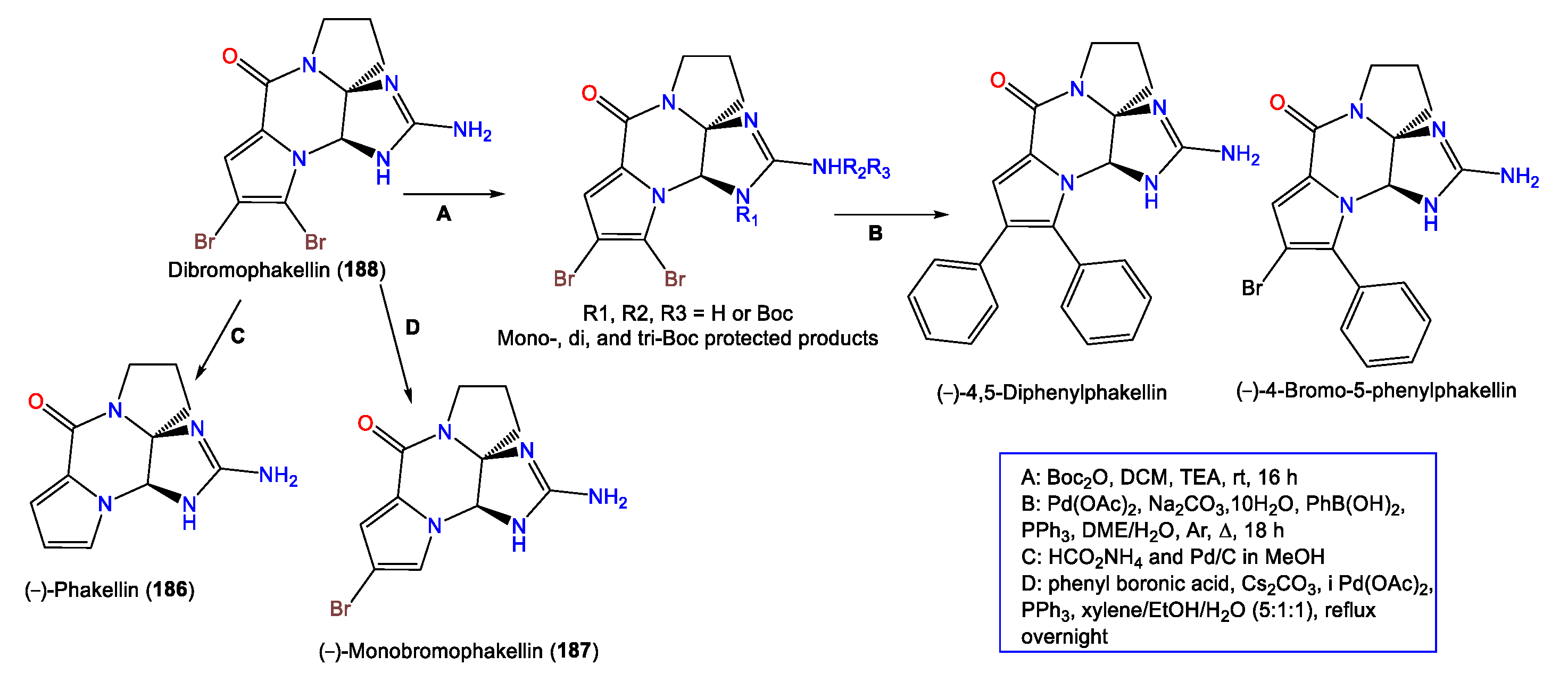
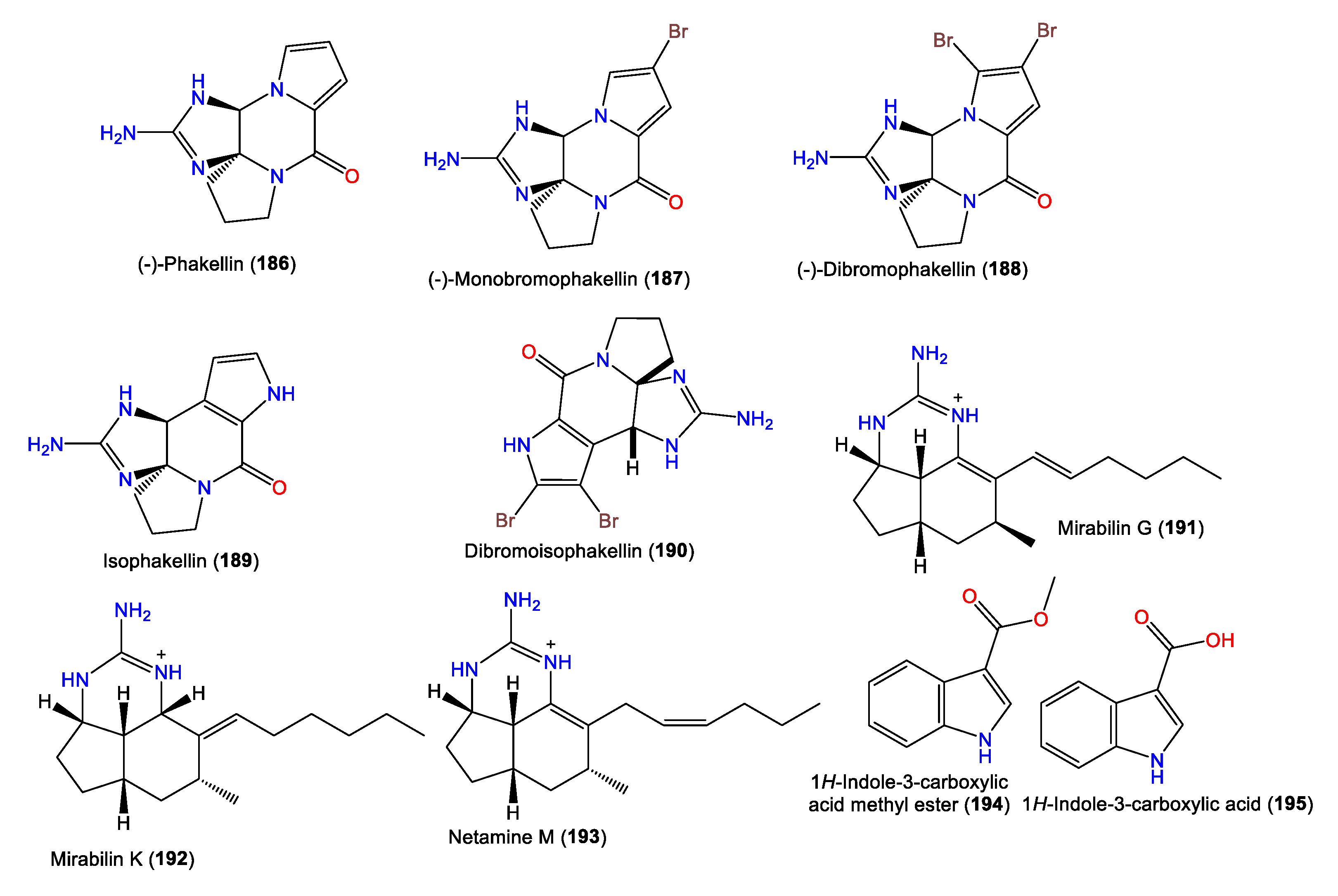
- Davis, R.A.; Fechner, G.A.; Sykes, M.; Garavelas, A.; Pass, D.M.; Carroll, A.R.; Addepalli, R.; Avery, V.M.; Hooper, J.N.; Quinn, R.J. (−)-Dibromophakellin: An α2B Adrenoceptor Agonist Isolated from the Australian Marine Sponge, Acanthella costata. Bioorg. Med. Chem. 2009, 17, 2497–2500.
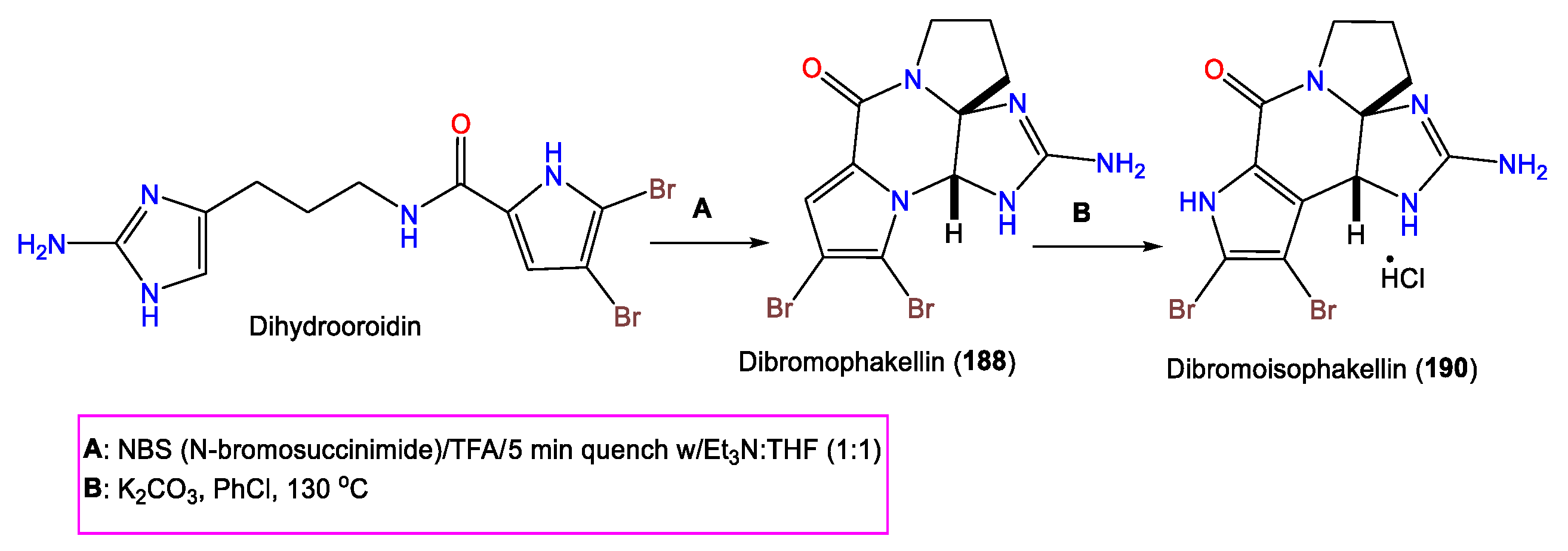
- Fedoreyev, S.A.; Utkina, N.K.; Ilyin, S.G.; Reshetnyak, M.V.; Maximov, O.B. The Structure of Dibromoisophakellin from the Marine Sponge Acanthella carteri. Tetrahedron Lett. 1986, 27, 3177–3180.
- Wiese, K.J.; Yakushijin, K.; Horne, D.A. Synthesis of Dibromophakellstatin and Dibromoisophakellin. Tetrahedron Lett. 2002, 43, 5135–5136.
- Grkovic, T.; Blees, J.S.; Bayer, M.M.; Colburn, N.H.; Thomas, C.L.; Henrich, C.J.; Peach, M.L.; McMahon, J.B.; Schmid, T.; Gustafson, K.R. Tricyclic Guanidine Alkaloids from the Marine Sponge Acanthella cavernosa that Stabilize the Tumor Suppressor PDCD4. Mar. Drugs 2014, 12, 4593–4601.
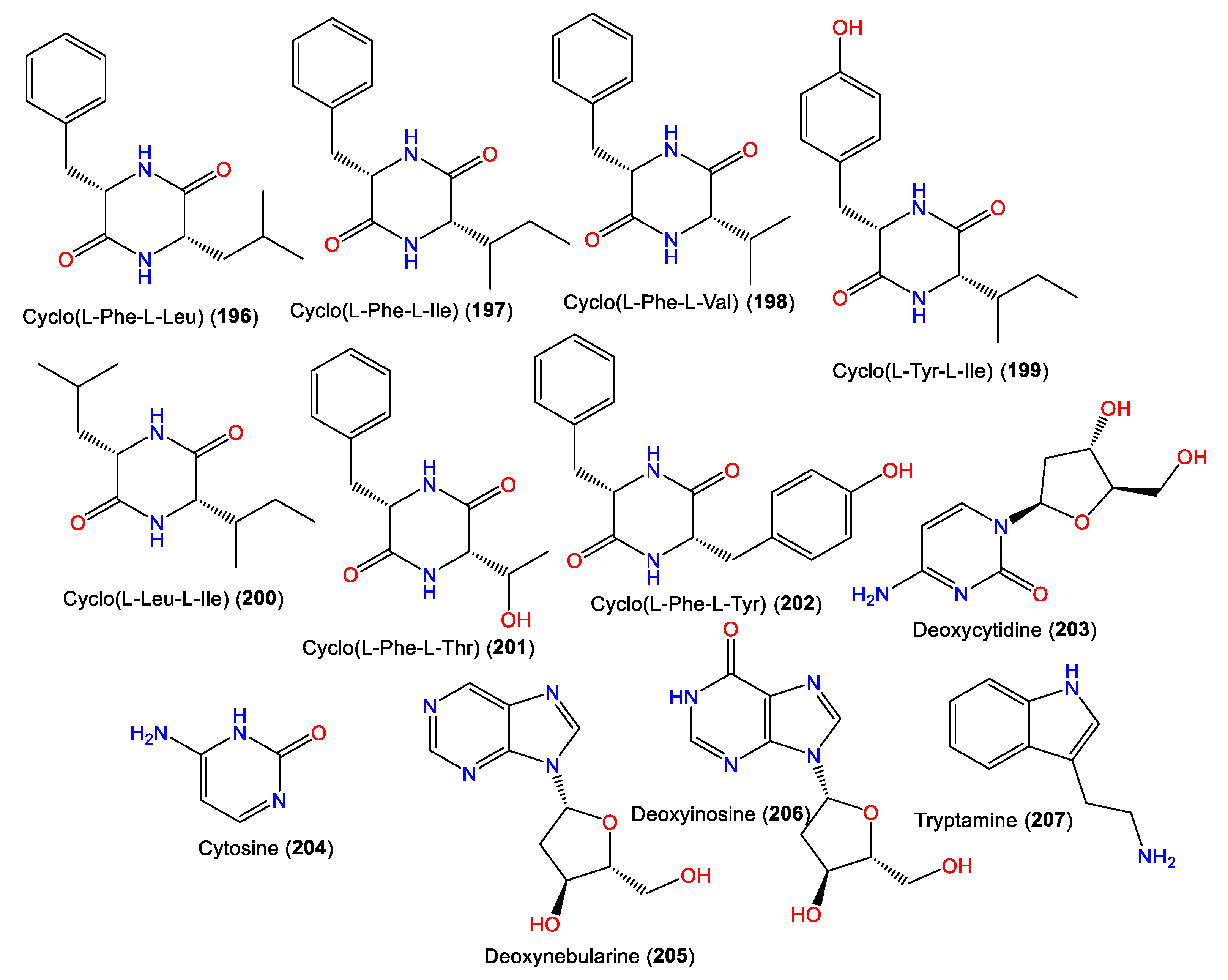
- Laville, R.; Nguyen, T.B.; Moriou, C.; Petek, S.; Debitus, C.; Al-Mourabit, A. Marine Natural Occurring 2, 5-Diketopiperazines: Isolation, Synthesis and Optical Properties. Heterocycles 2015, 90, 1351–1366.
- Qiu, Y.; Deng, Z.W.; Xu, M.; Li, Q.; Lin, W.H. New A-nor Steroids and their Antifouling Activity from the Chinese Marine Sponge Acanthella cavernosa. Steroids 2008, 73, 1500–1504.
- Tanaka Yoshito; Ito Yoshihito; Katayama Teruhisa. The Structure of Isoagelaxanthin a in Sea Sponge Acanthella vulgata. Bull. Jpn. Soc. Sci. Fish. 1982, 48, 1169–1171.
- Mancini, I.; Guella, G.; Pietra, F.; Amade, P. Hanishenols A-B, Novel Linear or Methyl-Branched Glycerol Enol Ethers of the Axinellid Sponge Acanthella carteri (= Acanthella aurantiaca) from the Hanish Islands, Southern Red Sea. Tetrahedron 1997, 53, 2625–2628.
