Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
A promoter is the region of genomic DNA located upstream of a gene that initiates the process of transcription under specific cellular conditions. Structurally, it is modular in nature and comprises a core promoter that includes a TATA box and a CAAT box, as well as proximal and distal regions.
- synthetic promoter
- CRISPR
- Cis-engineering
- transcription factors
1. Introduction
Genetic engineering has emerged as a promising molecular discipline that can efficiently resolve the existing agricultural problems related to the escalating global population and can boost the agricultural productivity. Assuming that the success of transgenics depends on the expression level of the transgene, researchers in the field of plant synthetic promoter engineering are striving towards the synthesis of robust, constitutive, inducible, and bidirectional synthetic promoters. These promoters will enhance the transcriptional regulation of transgene expression in model plants and agricultural crops [1,2,3,4,5,6].
The Cauliflower Mosaic Virus 35S (CaMV35S) promoter has been extensively employed in plant molecular biology-based research ever since it was discovered [7,8,9,10,11,12,13,14,15,16]; still, it is regarded as a ‘dark horse’ in this field. With the gradual expansion of transgenic research, there has been an increased demand for a versatile promoter with a higher transcriptional activity. Diverse members of the family Caulimovirideae were reported to contain useful native promoters, such as the mirabilis mosaic virus (MMV) [17], figwort mosaic virus (FMV) [18], soybean chlorotic mottle soymovirus (SbCMV) [19], dahlia mosaic virus (DMV) [20], rice tungro spherical virus (RTSV) [21], tobacco vein clearing virus, (TVCV) [22], horseradish latent virus (HRLV) [23], petunia vein clearing viruses (PVCV) [24], and cassava vein mosaic virus (CVMV) [25]. Nonetheless, the available number of native promoters is insufficient to meet the increasing demand in plant biotechnology. Moreover, native promoters are neither constitutive/tissue-specific/inducible nor bidirectional in nature [26,27].
Owing to the above limitations of native promoters, researchers are engaged in designing and testing synthetic promoters capable of pursuing gene transcription in a controlled fashion, depending on the environmental stimuli. In the last two decades, redesigning promoter structure by “Cis-rearrangement” and “shuffling of sub-domains” has acquired importance in maintaining robust gene expression in plants against abiotic and biotic stresses. [10,28,29,30,31,32,33,34,35,36,37]. Such a genetically manipulated transcriptional unit is constructed by employing several state-of-the-art molecular strategies, including promoter DNA shuffling, hybridization, domain exchange, domain duplication, site-directed mutagenesis, etc. [9,10,38]. The overall purpose of these approaches is to manipulate the architecture of the Cis-element present in the promoter DNA’s backbone, in order to generate a synthetic module with altered Cis-clouding that mainly facilitates their interaction with the cognate transcription factor (TF), leading to a different module with specific functionality. Such recombinant/chimeric promoters are gaining high popularity in the plant biotechnology arena, for boosting the gene expression in plants [9].
2. Promoter Structure and Function
A promoter is the region of genomic DNA located upstream of a gene that initiates the process of transcription under specific cellular conditions. Structurally, it is modular in nature and comprises a core promoter that includes a TATA box and a CAAT box, as well as proximal and distal regions [39] (Figure 1). Functionally, promoters play a central role in regulating gene expression by their combinatorial interaction between various Cis-regulatory elements such as ‘silencers, repressors, insulators, enhancers’, and different TFs, as depicted in Figure 1 [10,40,41,42].
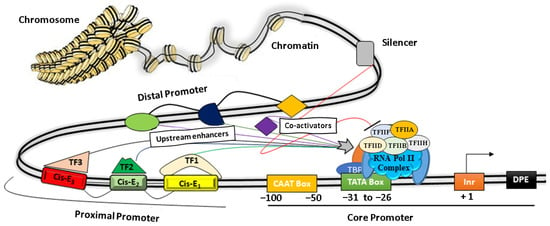
Figure 1. The basic architecture of plant promoters. The core promoter consists of a CAAT box, TATA box, Initiator element (Inr), and a downstream promoter element (DPE). Protein factors (TF IIA, IIB, IID, IIH, IIE, IIF, etc.) near the TATA box facilitate the binding of RNA Pol II to initiate transcription. The proximal regions (~1000 bp) and the distal promoter regulate the promoter activity by recruiting transcription factors (TF) on discrete Cis-elements (Cis-E).
The core promoter covers nearly 100 bp upstream of the TSS, whereas a proximal promoter extends up to a few hundred bp from the core promoter and contains several TFs binding sites [43]. The core domains consist of a conserved TATA box and motifs such as the CAAT box, GC box, Inr, and DPE. More specifically, the CAAT box recognizes and binds additional co-factors of RNA polymerase II complex such as TFIIB, TFIID, TDIIF, and TFIIE, whereas the GC box binds to various trans-activators for transcription initiations, as depicted in Figure 1. The Initiator element (Inr) is the part of the core promoter that directs the transcription initiation in typical conditions when the TATA box is dysfunctional. It has a YYANWYY consensus sequence that facilitates the binding of TF-IID and helps in strengthening the promoter [44]. Other protein factors bind to the downstream of a gene, such as Inr and regulate gene expressions [10] (Table 1, Figure 1). Several studies have shown that the proximal promoters (~1–2 kb upstream from the transcription start site) contain various important Cis-elements that strongly influence gene expression by recruiting TFs under specific cellular or environmental conditions [45,46,47]. For instance, the proximal sequence of the DREB2C promoter is sufficient for the tissue-specific spatiotemporal gene expression by heat stress in Arabidopsis [47].
Similarly, it has been shown that the simultaneous mutation at five protein binding sites (−410/−86) in the glutelin gene promoter leads to the elimination of full-length (1.8 kb) promoter activity [48]. Also, the minimal promoter proximal unit (86 to +217) of the tobacco late pollen gene g10 is sufficient for its expression in mature pollen [49]. The above studies show that the proximal regions contain crucial Cis-elements, and modulation of these elements leads to strongly altered promoter activity.
The distal promoter is the distant portion of the promoter ranging several kb upstreams of the proximal promoter and contains additional Cis-regulatory elements that have a weaker influence than the proximal promoter. Additionally, the distal regions mostly contain various Cis-elements and discrete DNA motifs (enhancers and insulators) that specifically recruit different TFs [50]. In particular, the enhancers are short DNA motifs bound with activators and they help the transcription initiation complex for enhanced gene expression. In contrast, insulators recruit repressors protein complexes to minimize or block the enhancer activity [40,51]. The downstream promoter element (DPE) recruits TF-IID at the core promoter with the help of the initiator (Inr) in the absence of the TATA box (Figure 1). Several TFs bind with Cis-motifs along with other protein factors that help the genes in enhancing/suppressing their transcriptional activity by altering the chromatin structure to facilitate/prevent the binding of the RNA polymerase complex [52,53]
Table 1. List of various Cis-motifs and trans-factors in the promoter complex.
| Promoter Cis-Motifs and Trans-Factors | Function | Reference |
|---|---|---|
| CAAT Box | Determine the efficiency of the promoter | [50] |
| GC Box | Bound by SP1 transactivator and related transcription factors | [54] |
| TATA Box | Binding site for RNA polymerase II | [50] |
| TSS/Inr | Beginning of transcription | |
| TFIIA | Promote the binding of TBP to the TATA box | |
| TFIIB | Couples to TFIID/TFIIA complex and Brings RNA polymerase II to the core promoter. | |
| TFIID | Adheres to core promoter | |
| TFIIE | Bind to the polymerase/promoter complex | |
| TFIIF | Tightly binds to the RNA Pol II | |
| TFIIH | Bind to the polymerase/promoter complex | |
| BRE | TFIIB binding sequence | |
| MTE | Motif Ten element; functions cooperatively with the Inr and is a recognition site for TFIID | [55] |
| DPE | Downstream promoter element; Recognition site for TFIID in Drosophila | [56] |
| TAFs | TBP associated factor, subunits of TFIID that assist TBP binding to DNA | [57] |
| TBP | TATA-binding protein, subunit of TFIID | [58] |
| Y Patch | Located between the TATA boxes and the TSS | [59,60] |
| CpG Island | Sp1 recognition site and simplify the regulation of gene activity by DNA-methylation | |
| I box | Light-responsive element | [61] |
| G-box | Associated with floral and root-specific expression | [60] |
| H-box | Associated with floral and root-specific expression | [60] |
| W Box | Play a role in systemic acquired resistance | [62] |
| S Box | Directs expression by fungal elicitors | [28] |
| CCAAT element | Act cooperatively with heat shock promoter elements (HSEs) | [63] |
| PB element | Potential overlap with WRKY and TGA binding | [40] |
| GCC Box | Regulation of jasmonate-responsive gene expression | [64,65] |
| HSRE | Up-regulation during the hypersensitive response | [41] |
| PC4 | Cofactor that interacts with VP16 TAD | [65] |
| SAGA | Histone acetyltransferases recruited by VP16 TAD | |
| PCAF | Play distinct roles in transactivation by MyoD | |
| UAS | Tends to contain primary regulatory elements | [66] |
3. Need of Developing Synthetic Plant Promoters
As discussed before, the major lacuna associated with the endogenous/native promoters is the weak transcriptional levels achieved within the host. Synthetic promoters offer significant ways for overcoming this obstruction and facilitating the desired gene expression, thereby allowing the efficient optimization of transgene expression at an appropriate time, place, and level [42,63]. The significant advantage of designing synthetic promoters is that it alters the promoter strength either by enhancing or reducing the promoter activity by rearranging Cis-elements via altering their copy number and spacing, which is ultimately reflected in their temporal and spatial expression pattern [27,53]. Additionally, synthetic promoters can minimize undesirable expression and remove other elements that may give rise to unwanted expression characteristics [53]. Overall, the significant role that is anticipated by synthetic promoters is to increase the target gene expression and control the unwanted and leaky expression to improve the precision and strength of the promoter [63,67]. Another objective of designing synthetic promoters is to establish sequence heterogeneity among them, which provides an added advantage for gene stacking/gene pyramiding approaches to achieve the simultaneous expression of multiple genes by avoiding promoter DNA homology-based combinations [10].
Depending upon the need of plant molecular biology, various environmental stimuli-specific synthetic promoters have been developed. [9] We described the different types of synthetic plant promoters as well as the responsible host. Our earlier review [9] detailed the different synthetic plant promoter types. In the present review, we discuss the latest advancement and development of such promoters over the past decade, along with the corresponding Cis-element and its nature; the responsible host is represented in an informative way in Table 2. Advancements made in the field of synthetic promoters are presented below.
3.1. Biotic Stress-Inducible Synthetic Promoters
The main role of a pathogen-inducible promoter is to provide resistance to a wide range of pathogens [68]. Lin et al. developed an 868-bp pathogen-inducible promoter PLsGRP1 from Lilium, which showed high activity on the pathogen and cold stress. LsGRP1 is a defense-related leaf-specific gene against a fungal pathogen causing gray mold disease. The 131-bp 3′-end region of PLsGRP1 also showed activity against various biotic, abiotic, and phytohormone exposure [69]. Four pathogen-inducible Cis-regulatory elements (PICEs) were identified in rice, namely AS-1, GCC-box, G-box, and H-box. About 53.5% of PICEs showed up- or down-regulation when exposed to pathogen attack [70].
3.2. Abiotic Stress-Inducible Synthetic Promoters
Crop productivity is majorly affected by various abiotic factors, among which high salinity and drought are the major ones. Moisture deficiency critically checks plant growth and poses constraints to crop production worldwide. This necessitates the construction of salt- and drought-inducible promoters, which can possess an optimum affinity to such stresses and can be used for imparting increased levels of tolerance to either one or both of these two abiotic stress agents. Chen et al. identified the OsHAK1 promoter sequence of 3037 nt in rice, which was found to activate under drought stress [71]. The stress-responsive gene gNAC21 was characterized from pearl millet (Pennisetum glaucum) and was reported to induce salinity tolerance in various crops [72].
3.3. Chemical-Responsive Synthetic Promoters
Certain chemicals have also been reported to regulate the transgene expression by enhanced transcription. These chemicals include various antibiotics, ethanol, herbicides, insecticides, etc. Chemically induced synthetic promoters were developed by employing probenazole, a chemical inducer which induces salicylic acid (SA) biosynthesis by inducing (SAR) systemic acquired resistance in plants [73]. A tetracycline-inducible system was developed for tobacco BY-2 suspension cells [74]. Alcohol-inducible gene expression system (AlcR-PalcA) has been successfully used in various plants such as Arabidopsis thaliana [75], Lycopersicon esculentum (tomato), and Populus sp. [76,77]. AlcR-PalcA has also been reported in microalgae Chlamydomonas reinhardtii. The alcohol-inducible AlcR-PalcA system originates from Aspergillus nidulans, a filamentous fungus [78]. A list of agrochemicals that can be used as chemical inducers is also available [79].
3.4. Development of Hormonal-Responsive Synthetic Promoters
The transcriptional regulation of different promoters is a complex process heavily influenced by several plant hormones. Wu et al. reported that the spatial pattern of abscisic acid (ABA) mediated transcriptional regulation by evaluating an ‘abscisic acid responsive element’ (ABRE) in root tissues [80]. The interaction of the synthetic promoter in association with functional genes responsible for cytosolic ABA, receptor kinase 1 (CARK1), and regulatory components of ABA receptor 11 (RCAR11) lead to drought stress tolerance [81].
3.5. Constitutive Promoters
Constitutive promoters continuously control the downstream gene expression, irrespective of space and time. To date, many promoters have been manifested with constitutive expression of genes, which have been reported to have low quality and poor yield [69]. The CaMV35SRNA promoter is the most common constitutive promoter used for various plant species, and other promoters include ubiquitin promoters [82].
3.6. Wound-Inducible Promoter
A wound-inducible promoter works exceptionally well to express the insecticidal gene in many crop plants. These promoters usually contain WRKY, W-box, and FORCA Cis-elements that recruit specific TFs for their action. Until now, several wound-inducible promoters have been isolated from different sources, namely OsDof1, Shpx6, win3.12, fib, mpiC, AoPR1, and RbPCD1 promoters [83,84,85,86,87]. Some of the above-listed promoters, such as AoPR1, mpiC1, and RbPCD1, have been used to derive CryIAc-type proteins with varying success rates [88,89]. Recently, PW220 and pRHA3B I promoters have been characterized and have been induced with wound-stress treatment in Arabidopsis [90,91]. These promoters could be used as excellent tools for developing insect-tolerant crop plants.
3.7. Bidirectional Promoters
Bidirectional promoters (BiP) are more pertinent than unidirectional promoters, as they can control the expression of two genes simultaneously, thereby saving time from expression vector construction and piling multiple genes [5]. Moreover, the limited availability of unidirectional promoters with the same expression pattern has also encouraged the application of bidirectional promoters [92]. Bidirectional promoters have been designed in many plant species such as Arabidopsis [93], rice [94], melon [95], and Capsicum annum [96], all of which have been successfully reported. In Zea mays, a novel gene stacking strategy was applied by combining a bidirectional promoter (BDP) with biCistronic approaches. This gene stacking configuration demonstrated the application of a single promoter for the coordinated expression of multiple genes in corn, a crop plant [97]. Recently, a CRISPR-Cas9 approach for genome editing in rice was investigated employing a BiP [39]. A BiP has also been used in microalga [98].
3.8. Chimeric/Hybrid Promoters
Chimeric/hybrid promoters are developed when a different functional part from one promoter is fused with the homologous/heterologous counterpart taken from another promoter. These Cis-modified promoters are developed for their utility in avoiding unpredictable and unwanted genetic recombination in plants. A new chimeric promoter was developed for controlling vascular pathogen infections by the fusion of the CaMV35S promoter and the xylogen protein 1 promoter (Px), where it serves as a vital factor in the development of the xylem in Arabidopsis [99].
3.9. Tissue-Specific Synthetic Promoters
Several tissue-specific promoters have been developed for regulating gene expression in a specific organ or tissue, such as root-specific [100], green-tissue specific [101], endosperm-specific, pollen-specific [102], etc. Green tissue-specific promoters were developed in switchgrass (Panicum virgatum L.) to enhance the biofuel production and to reduce cell wall resistance without altering the root phenotypes [103]. Recently, bidirectional green tissue-specific promoters (BiGSSP2, BiGSSP3, BiGSSP6, BiGSSP7) have been constructed [104].
Table 2. List of synthetic promoters.
| Synthetic Promoter |
Cis-Element Involved | Nature of the Promoter | Host Organism Tested |
Reference |
|---|---|---|---|---|
| SynP16 | Multiple combinations of soybean ABF, ABRE, ABRE-Like, CBF, E2F-VARIANT, G-box, GCC-Box, MYB1, MYB4, RAV1-A, and RAV1-B. Minimum 35S core promoter | Multiple expression | Glycine max | [105] |
| 12–10, 12–48, 12–79 |
Skn-1 motif, HD Zip/WUN, ABRE/boxii/Ace, CGTCA/TGACG-motif, O2 site, caat box, G box, AAGAA-motif, TATA box | Constitutive expression | Physcomitrella patens | [106] |
| AZprom (1–21) | Camv35s and Ribulose-1,5-bisphosphate carboxylase small subunit promoter | Constitutive expression | Nicotiana tabacum var Samsun | [107] |
| Saps (Sap-11) | GC content, AT and TC rich motifs, POWRS motifs | Constitutive expression | Chlamydomonasreinhardtii | [108] |
| TGA1 (CmYLCV) |
Cis-elements of CmYLCV (cestrum yellow leaf curl virus) at distal promoter region. | Constitutive expression | N. tabacum, Arabidopsis thaliana | [109] |
| SynS1, SynS2 | Various Cis-elements in the synthetic module (SynS) | Constitutive expression | Saccharum officinarum | [110] |
| Ap, Dp, ANDp | The sequences of RD29A and RD29B promoters as promoter sequences and as Cis-elements. | Inducible expression | A. thaliana | [81] |
| P_DRE:35S | Core element of camv35s promoter and tmv omega 5′-UTR, 35S core sequence. | Inducible expression | A. thaliana | [110] |
| SINC, GmubiSINC | 5′ UTR of soybean polyubiquitin promoter, Upstream camv35s | Inducible expression | Glycine max | [111] |
| SP-DDEE | e17 elements and minimal promoter, Parsley-D | Inducible expression | Brassica napus | [112] |
| GWH | JERE, SARE, GCC, 2x HSRE and 6x W-box | Inducible expression | A. thaliana | [73] |
| MAMP | Minimal promoter, uidA reporter | Microbial pathogen attack | Petroselinum crispum | [113] |
| Pmec, Pcec, Pdec and Ptec | TAM(transcription activation module), Pmec (minimum cassette expression) | Enhanced expression | Solanum lycopersicum | [16] |
| 4 x CCTC | Pi transporter 3 (StPT3) | Inducible expression under low Pi condition | Rizhophagus irregularis | [114] |
| 4 x GCC-box motifs | AtPDF1.2 promoter | Inducible expression Jasmonic acid (JA) | A. thaliana | [115] |
| ROSE 1–7 | ROSE7/GCC-box, MPK6, ERF6 | ROS response during oxidative stress | A. thaliana | [116] |
| 4 x RSRE | RSRE:;LUC reporter, GSR-motif | Rapid stress response | A. thaliana | [117] |
| PRSGA, P2RSGA, P2RSPA, PRSGPA, P2RSGPA, PR5SGPA, P2R5SGPA | ACGT and AACA motifs, Skn-1 (S), Prolam in box (P), GCN4 (G), RY repeats (R), Pzmbd1 | Seed-specific bidirectional Promoters | Zea mays | [118] |
| BiGSSP2, BiGSSP3, BiGSSP6, and BiGSSP7 | Osactin (1), Ostubulin (6, 6i) previously reported sequences, Posrbcs-550, Posrbcs-62. | Bidirectional expression in green tissues specifically. | Oryza sativa | [104] |
| SynR2 SynR1 | SynR1—Synthetic module at 5’ end SynR2—Synthetic module at 5’ and 3’end |
Root specific | N. tabacum | [119] |
| GSSP1, GSSP3, GSSP5, GSSP6, GSSP7 | First intron in rice (Act1), GT and G box. Regulatory sequences of rice, tobacco and Arabidopsis (PD 500–540, Posrbcs-550, 62 and enp3-110) |
Green tissue-specific | Oryza sativa | [120] |
| MUASMSCP | Mmvflt and mmvsgt | Constitutive expression | N. tabacum, entire plant of Petunia hybrida and protoplasts of A. thaliana | [121] |
| FSgt-PFlt, MSgt-PFlt, PFlt-UAS-2X | UAS of sub-genomic promoter transcript of MMV and FMV coupling with peanut chlorotic streak virus | Constitutive expression | N. tabacum, Amaranthus, A. thaliana, Petunia hybrida and S. lycopersicum | [35] |
| FSuasFcp, FuasFScp | Cis-elements of FMV (Figwort mosaic virus)—Sub genomic and full-length transcript promoter. | Constitutive expression | N. tabacum | [122] |
| VR-ACS1 | 5’ UTR of Vigna radiata aminocyclopropane-1-carboxylate synthase | Constitutive expression | Vigna radiata | [123] |
| p4xKST82-rd29B | rd29b: Arabidopsis rehydration responsive promoter 4xkst82: Potato KST1 promoter |
Inducible expression | N. tabacum | [124] |
| GCC2X, GCC3X, W2X, S2X | Minimal promoter of camv35s at 46 regions 2–3 copies of W, GCC and S boxes in transgenic tobacco |
Inducible expression | N. tabacum | [125] |
| EKCRM, ECCRM, EKCM | Derivative of Arabidopsis rd29a, erd1, cor15a and kin1 sequences | Inducible expression | A. thaliana | [126] |
| EFCFS-HS-(1, 2, 3) | Derived from FUAS and FS3CP containing aaag Cis-motif (Dof-1) | Inducible expression | N. tabacum | [11] |
| SP’s, SP-(EE, FF, EEFF) | Upstream regions of camv35s, F and E17 | Inducible expression | B. napus | [127] |
| pporRFP | 4XRE (regulatory element) located between B (−415, −90) and A1 (−90, −46) promoter domains of camv35s | Inducible expression | N. tabacum cv. Xanthi | [128] |
| ACGT, ACGT-2, ACGT-N-(5, 10, 25) | Single/double copies of ACGT activator motif, Pmec minimal promoter |
Inducible expression | A. thaliana and N. tabacum leaves | [30] |
| p35S-PCHS-Ω, p35S-LCHS-Ω, pOS-PCHS-Ω, pOS-LCHS-Ω |
CHSA core promoter of Petunia, CHS core promoter of Lily, Ω element, camv35s/OCS enhancer region Petunia CHSA core promoter, Lily CHS core promoter, Ω element, camv35s or OCS enhancer region |
Flower specific expression | Torenia fournieri | [129] |
| FUASCsV8CP | Up-stream activation sequence of FUAS and cp Cassava Vein Mosaic Virus | Salicyclic inducible expression | N. tabacum | [37] |
| 4D, 2S2D | 4D, 2S2D, D-box (GGAACC), Box S(AGCCACC) | Elicitor-responsive | N. tabacum | [130] |
| MSgt-FSgt | UAS domain of mmvsgt and core domain of fmvsgt | Constitutive expression | N. tabacum, A. thaliana | [131] |
| FS3-UAS-3X, FS3-UAS-2X | Fmvsgt | Constitutive expression | N. tabacum cv. | [31] |
| FS-(TGACG), FS-(TGACG)2, FS-(TGACG)3 | TGACG motif of F-Sgt | Constitutive, salicyclic acid (SA) inducible | N. tabacum and A. thaliana | [31] |
| 2x::GUS, 4x::GUS, -148-3’::GUS,-137-3’::GUS | 57 bp fragment of atmyb60 promoter and camv35s promoter | Tissue-specific expression | A. thaliana | [32] |
| pCL, pLC | CRT/DRE element (CCGAC) and TSSR of Arabidopsis cor15a promoter and potato class I promoter | Tissue-specific, cold-inducible | S. tuberosum | [132] |
| mPtDr102 | Methyl jasmonate inducible synthetic promoter derived from ptdr102 and camv35s | Bidirectional, methyl jasmonate inducible | N. tabacum | [133] |
| pOCSn-OCS, pLOCSn-OCS, pΔOCS, pLOCSΔOCS | Ocs element of octopine synthase promoter | Constitutive expression | N. tabacum | [134] |
| FUAS35SCp, MUAS35Scp | Fmvflt, mmvflt and camv35s | Bidirectional, constitutive | N. tabacum | [34] |
| A27znGlb1 | 27zn and Glb1 promoter | Tissue specific expression | Zea mays | [135] |
| 2 X W2/2 X S/2 X D, 4 X W2/4 X S | W1, W2, GCC, JERE, S, Gst1, and D boxes. CaMV35S minimal promoter | Inducible expression, Pathogen attack | Parsley | [28] |
| 2xABRE, 4xABRE | Wheat Em gene promoter | Salinity/abscisic acid inducible | Rice plant (elite Indica rice variety Khitish) | [136] |
This entry is adapted from the peer-reviewed paper 10.3390/agriculture13020298
This entry is offline, you can click here to edit this entry!
