1. Overview
Although their usefulness in clinical practice is poorly understood, the use of lncRNAs as predictive biomarkers in response to therapy has advantages compared to protein-based and mRNA-based biomarkers [
39] since they present tissue, and stage specific expression [
40]; this gives them greater sensitivity and specificity [
41], particularly in tumors with hormone sensitivity, such as the prostatic adenocarcinoma, in which some lncRNAs with clinical utility, such as
SChLAP1 [
42],
lncRNA-p21 [
43], and
PCA3 [
44], have been identified. As their association with prostate cancer has already been established, this allows for their use in clinical practice. For example,
SChLAP1 is a lncRNA whose length is 854 nt, is transcribed from chromosome 2, and is differentially expressed in bladder normal tissue and prostate cancer tissue. It was first identified in paraffin-embedded tissue biopsies by
in situ hybridization (ISH). The biological function of
SChLAP1 is related to the regulation of the SWI/SNF chromatin-modifying complex; this lncRNA antagonizes the genome-wide localization of this protein complex, which is related to the promotion of invasiveness and metastasis in LNCaP and 22Rv1, as well as in Du145 cancer cell lines [
45]. Moreover,
SChLAP1 expression has also been associated with metastasis (odds ratio [OR] 2.45, 95% CI 1.70–3.53;
p-value < 0·0001) and cancer progression (hazard ratio = 1.99,
p-value = 0.032) in prostate cancer patients [
46]. Additionally,
lincRNA-p21, which is a lncRNA, has been shown to be differentially expressed in prostate cancer [
47]; its biological function is principally the regulation of apoptosis, cell proliferation [
48], and cell cycle by its interaction with MDM2 and STAT3 [
47].
lincRNA-p21 has also been related to disease progression in prostate cancer in preclinical studies, as its overexpression in castration-resistant patients who were treated with enzalutamide is associated with less overall survival (
p-value = 0.04), which indicates that
lincRNA-p21 could also be a useful predictive biomarker for enzalutamide treatment [
47]. Finally, the Prostate Cancer Antigen 3 (
PCA3), a lncRNA of 3 Kb in length transcribed in chromosome 9, is present in prostate cancer with high tissue-expression specificity, described first by Bussemakers
et al. in 1999 [
49]. Currently,
PCA3 is also an auxiliary biomarker in prostate cancer; its use was approved by the Food and Drug Administration (FDA) in 2012 [
44] due to its clinical utility by reducing the number of unnecessary biopsies in patients. Moreover,
PCA3 has been reported to be related to the survival of prostate tumor cells by regulating the androgen receptor signaling pathway, as well as regulating the epithelial-mesenchymal transition (EMT) by modulating some targets, such as E-cadherin and TWIST [
50,
51]. Furthermore, it has been used in gene signature PROGENSA to determine which patients with a previous negative biopsy [
52] need a second biopsy [
53]. As described above, the use of molecular biomarkers based on lncRNA expression for prostate cancer has demonstrated the utility of this RNA biotype in clinical practice. Likewise, this could be extended to breast cancer clinical application since both carcinomas are characterized as hormone-sensitive [
54], and there is experimental evidence of lncRNA expression related with clinical outcome, such as lincRNA-ROR, in which PCA3 regulates EMT by modulating E-cadherin functions [
55]. Thus, it is necessary to implement more research to have similar results in biomarker discovery for breast cancer.
On the other hand, in prostate cancer research, it has been established that, although the lncRNA expression itself has clinical utility, the identification and detection of different biotypes, such as mRNAs, and genetic fusions also has utility in clinical practice [
56]. Indeed, there are reports in scientific literature that demonstrate that the combination of lncRNA, mRNA, and genetic fusions in molecular signatures has improved the sensitivity or specificity of assays based priorly only in the expression of one gene [
57]. One example is PROGENSA, which is based on
PCA3 expression and is associated with a sensitivity of 66–72%, and a specificity of 58–76% [
58,
59], while Mi Prostate Score, an urinary test based on the detection of PSA (mRNA),
PCA3 (lncRNA), and TMPRSS2-ERG (genetic fusion) [
60], has an associated sensitivity value of 95%. This demonstrates that the combinatorial use of mRNAs, lncRNAs, and genetic fusions can improve the results of laboratory tests for prostate cancer, and this could be extended to breast cancer research.
2. Challenges and Perspectives for lncRNA Clinical Application as Predictive Biomarkers for Breast Cancer Management
For breast cancer, there are few studies that support the use of lncRNAs or the combination with other biotypes as molecular predictive or prognostic biomarkers in clinical practice, and none of them have been approved for commercial distribution, as in the case of PROGENSA, although there is already evidence in scientific literature about their potential as biomarkers in decision-making for the management of breast cancer patients [
61,
62,
63]. The best example to describe the potential clinical utility of a lncRNA in patients with breast cancer is the study performed by Berger
et al. in which the existence of lncRNA-coding gene regulation networks, such as
NEAT1,
TERC, and
TUG1, together with other mRNAs, such as ESR1, AR, and SOX2, make it possible to classify patients with gynecological cancers and breast cancer into 6 clusters, which are related directly to their phenotypes and mainly to the immune response, as well as to the expression of hormone receptors in patients particularly associated with the estrogen receptor signaling pathway. This biomarker can be used for diagnostic, predictive, and prognostic purposes in breast cancer patients [
64]. Furthermore, this has also been demonstrated by Niknafs
et al., who described the use of
DSCAM-AS1 expression as part of the characteristics of luminal tumors that are positive to hormone receptor expression [
13]. It has also been described by Contreras-Espinosa et al. for
GATA3-AS1 [
17], and for the LINC01087, which expression profile is also related with luminal phenotypes in breast cancer [
65]. This suggests that
GATA3-AS1 expression may be a relevant molecular characteristic that defines luminal tumors [
66]. However, there are additional emerging lncRNAs that have been described as potential biomarkers in cancer, such as
HOTAIR,
DSCAM-AS1, and
GATA3-AS1 in breast cancer [
8,
13,
17],
MALAT1 in lung cancer [
67],
H19 in colorectal cancer [
68],
HULC in liver cancer [
69],
UCA1 in bladder cancer [
70], and
DLEU1 in endometrial cancer [
71]. Among other lincRNAs [
72], the applicability of lncRNAs in the molecular diagnostic area and their use in laboratory tests for clinical diagnosis in the near future largely depends on the expansion of knowledge about their association with different clinical variables, such as response to treatment and overall survival, as well as their inclusion in clinical trials in order to determine and validate the benefits of their use in clinical routine, as it has been made for coding genes before [
73]. Thus, there are still many studies to be carried out in order to include lncRNAs more frequently in laboratory tests for the patient’s workup and treatment, not only in oncology, but also for other pathologies, like cardiovascular diseases [
74], and diabetes [
75], which are also leading causes of morbidity worldwide [
76]. Taken together, these results suggest that lncRNAs may be relevant biomolecules that could allow oncologists to differentiate patients who do not respond to therapy, regardless of the molecular heterogeneity of breast tumors, which represents an important challenge in oncology practice [
77].
3.The Use of lncRNAs as Molecular Biomarkers in the RNA-Based Therapeutics Era
A molecular feature advantage that distinguishes lncRNAs is their stability in biological samples, such as blood, urine, or saliva (median half-life ~3.5 h) [
78]. This is due to their transport in exosomes, microvesicles, apoptotic bodies, high density lipoprotein capsules, or into circulating tumor cells [
79], in contrast with mRNAs, which are characterized by their instability in body fluids (median half-life < 2 h) [
80]. This allows the detection of lncRNAs by non-invasive techniques through the use of liquid biopsies, such as urine and saliva, and less-invasive methods, such as serum and plasma [
40], as has been reported for lncRNAs
HOTAIR [
8] and
H19 [
9] in breast cancer, as well as for
MALAT1, which has been shown to be a serological marker in breast cancer [
36] and a diagnostic biomarker for oral squamous cell carcinoma that can be detected by saliva testing [
81]. The detection of these lncRNA is performed by a quantitative polymerase chain reaction (qPCR) in RNA extracted from serum or saliva obtained from patients. Likewise, it is possible to detect lncRNAs with the use of other techniques with higher sensitivity, such as ISH-RNA, which has been used for the rapid detection of markers, such as HER2 in breast cancer, [
82] with greater sensitivity and specificity (99% and 98%, respectively) when compared to HER2 immunohistochemistry (IHC) assay detection (95% and 98%, respectively) [
83]. The ISH-RNA assay has also allowed the detection of lncRNA
SNHG3 as a potential diagnostic biomarker, distinguishing between normal breast tissue and cancerous breast tissues [
84]. Furthermore, there are novel molecular approaches, such as spatial transcriptomics, which allow for the identification of a signature based on 798 transcripts, including the lncRNA
LINC00657, that could be implemented in machine learning methods to distinguish invasive breast cancer [
62]. In summary, the implementation of molecular biology techniques for lncRNA-based biomarkers detection in clinical practice could improve the reliability of the results of laboratory tests and the accuracy of oncological diagnosis.
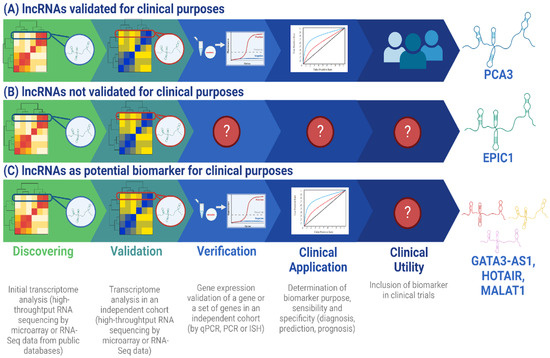
As discussed above, the implementation of
PCA3,
DSCAM-AS1, and
GATA3-AS1 as other lncRNA molecular biomarkers represents a novel approach for the clinical management of the oncological patient (
Figure 1) since their expression is tissue-specific, disease-specific, and is associated to a particular stability in body fluids [
2], contributing to the development of precision medicine; this is because lncRNA-based biomarkers offer simple and reliable tests [
85]. Altogether, this represents the lncRNA-based diagnostics [
86,
87], a new concept in medicine which integrates the potential use of lncRNAs as molecular biomarkers, with application in clinical practice, that will improve patient management in three main aspects. The first is the use of non-invasive techniques for laboratory tests (e.g., liquid biopsies); this has proven to be useful in clinical routines as the urine analysis, which is currently in practice with the use of
PCA3 [
88]. The implementation of these molecular assays, with fluids like urine and saliva, have the main objective of benefitting patient management because these methods allow the oncologists to perform the diagnostic and follow up of patients in less invasive manners, with the accuracy improved, due to the capability of these non-invasive methods to avoid some bias, like tumor cell heterogeneity [
89]. Hence, the detection of lncRNAs by the implementation of non-invasive methods, such as urine and saliva analysis, is a promising improvement in clinical routine. Second, the use of time and cost-efficient detection techniques, such as qPCR, which take ~2 h to get results [
90] in contrast to IHC, which takes approximately 2 days or more [
91], will directly impact the optimization of the oncologist decision making, for example, in the decision for treatment selection for breast cancer patients. Third, the improvement in result accuracy for laboratory tests. Because of the high specific expression profile of lncRNAs, as well as their sensibility and specificity, differential diagnosis and early diagnosis are easier and can also be combined with pathological imaging processing that involves the use of X-ray imaging, magnetic resonance imaging, nuclear medicine imaging, and ultrasound imaging, which are techniques with routinary use in clinical practice [
92]. Thus, the combinatorial use of molecular and image biomarkers could lead to the improvement in diagnosis, prediction, and prognosis values [
93], which have been demonstrated by the implementation of machine learning algorithms for the integration of molecular imaging and clinical data [
94,
95,
96]. However, to achieve the implementation of combinatorial biomarkers in breast cancer, the development of appropriate research protocols is necessary to demonstrate and validate their usefulness in clinical practice.
Figure 1. Workflow for lncRNA validation as biomarkers with clinical utility and application. The process of implementing a lncRNA-based biomarker consists of 5 principal steps: discovery, validation, verification, clinical application, and clinical utility [
97]. In the discovery step, the objective is to select a lncRNA (or a set) differentially expressed in the condition of interest, like treatment response, and implement it in selected patients. In the validation step, the sample size is increased to determine lncRNA robustness to define the clinical condition of interest and follow the sample size calculation recommended in [
20]. In the verification step, lncRNA expression is determined by a clinical laboratory technique, such as qPCR, to verify its viability to be detected in the clinical routine. In the clinical application step, the functionality of the lncRNA as a biomarker for diagnosis, prediction, or prognosis is determined by assessing its sensibility and specificity [
20]. Finally, in the clinical utility step, the accuracy of the lncRNA as biomarker is tested in a larger sample size and could be included in clinical trials. (
A)
PCA3 is an example of a lncRNA that has been validated for clinical application in prostate cancer diagnosis because it represents an FDA-approved lncRNA for clinical purposes. It was discovered from a sample size of 11 patients in the discovery phase [
49] and 507 male patients were included in the validation phase in a clinical trial [
53]. Additionally,
PCA3 is associated with a sensitivity ranging from 54% to 82% and a specificity range of 56.3% to 89%, which justifies its use in clinical practice [
50]. Although
PCA3 was identified by Northern blot technique [
49], it has been validated in other studies by high throughput sequencing technologies, which is the principal tool for current biomarker discovery [
98]. (
B)
EPIC1 is a lncRNA that was identified from the analysis of 6475 tumor samples in the discovery phase and 534 samples in the validation phase [
99]. However, it has not yet been verified as a biomarker for clinical utility or for clinical application in the prognosis of breast cancer. (
C)
GATA3-AS1 is a lncRNA proposed as a potential clinical biomarker in predicting treatment response in breast cancer because it has been demonstrated by Zhang et al. that it is overexpressed in breast neoplasia in a differential expression analysis for 85 paired tumor-normal samples and 830 tumor samples in the discovery phase. For the validation step, 50 paired tumor-normal samples and 23 healthy samples were included [
14]. Recently, Contreras-Espinosa et al. also identified this lncRNA by a machine learning approach in a sample size of 11 patients for the discovery step and 68 patients for the validation step, which demonstrated its utility as a predictive biomarker [
17]. However, it has not been validated for clinical application yet, as other lncRNA which have been proposed as molecular biomarkers for treatment response prediction in breast cancer, like
HOTAIR [
8] and
MALAT1 [
36], have not been included in clinical trials for the analysis of their applicability in diagnosis. lncRNA: long non-coding RNA; ISH: in situ hybridization; PCR: Polymerase Chain Reaction; qPCR: quantitative PCR. Created in BioRender.com.
4. The Current Challenges for lncRNA Research and for Their Implementation as Molecular Biomarkers in Routine Clinical Practice
Finally, it should be noted that the principal objectives in the investigation of the use of lncRNAs as current molecular biomarkers in breast cancer have mostly been aimed at determining the biological function of lncRNA [
100,
101] or their ability to describe mammary tumors molecularly, as is the case of lncRNA
EPIC1 described by Wang et al., which has been identified as an oncogene in breast cancer that promotes cell cycle and has been associated with poor overall survival (hazard ratio ~2,
p-value = 0.005) [
99]. However, it has not yet been possible to apply this knowledge in biomarker development for routine used in clinical practice, as it occurs with
PCA3 in prostate cancer [
44]. This happens because of three main reasons: (1) the sample size used for the discovery and validation of these potential biomarkers, (2) the lack of clinical trials focused on exploring the association of lncRNAs with clinical variables, and (3) the lack of clarity and accurate use of clinical definitions that involve biomarkers and clinical assessments, which make the development of new clinical tools based on novel molecular markers, such as lncRNAs, and their inclusion in clinical practice difficult [
102]. However, their advantages over other biomolecules, such as proteins and mRNAs, have been demonstrated [
40], as is the case of
GATA3-AS1, which predicts neoadjuvant chemotherapy resistance in luminal B-like breast cancer patients with a sensitivity of 92% and specificity of 75% (
p-value = 0.0001) [
17] compared to Ki-67, a clinical biomarker for neoadjuvant chemotherapy response prediction in breast cancer (sensitivity: 95.7%, specificity: 54.3%,
p-value = 0.002) [
103]. Another example is
H19 [
68,
104] and
DSCAM-AS1 [
13,
105]; not only has their biological function been described, but so has their applicability in clinical practice by establishing associations with clinical variables, such as estrogen receptor expression, which has potential application for diagnosis (sensitivity, 100.0%; specificity, 97.0%;
p-value < 0.001), as well as predictive and prognostic features [
105]. Furthermore, there are also studies for lncRNAs that are used as genetic signatures [
4,
14,
106]. Wang
et al. reported the use of a gene signature based on lncRNA expression that included
NEAT1, and its predictive value for neoadjuvant chemotherapy response was described (sensitivity, 69.9%; specificity, 77.8%;
p-value < 0.0001) [
106]. However, the sample size in these studies is small and this has not allowed for the scale up of the applications of lncRNAs to the dimensions of a clinical trial, as is the case of
H19 [
9],
HOTAIR [
8],
MALAT1 [
36], and
GATA3-AS1 [
17], which have predictive value for neoadjuvant chemotherapy response.
In resume, the new era of molecular diagnosis should consider the issues discussed above and, at least, allow for the reflection of three perspectives in its development. First, the integration of lncRNAs in clinical trials for the study and analysis of their potential applicability in clinical diagnosis. Second, the use of lncRNAs as diagnostic molecular biomarkers using non-invasive tests, as is the case for PCA3 detection in urine and for other promising lncRNAs, such as HOTAIR in serological tests and MALAT-1 in salivary tests, which represent an advance in the proper management of oncology patients, as we discussed before. Third, the integration of lncRNAs in commercial gene signatures and laboratory tests with diagnostic purposes, as was previously discussed, to improve the accuracy and reliability of diagnostic results, which will be reflected in the enhancement of oncology management strategies and in the amelioration of cancer patients’ quality of life. Finally, lncRNAs are part of the RNA world that have potential use as molecular biomarkers, which could be used in the near future as part of routine testing in breast cancer management.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24087426