Mitochondria are membrane-bound cellular organelles of high relevance responsible for the chemical energy production used in most of the biochemical reactions of cells. Mitochondria have their own genome, the mitochondrial DNA (mtDNA). Inherited solely from the mother, this genome is quite susceptible to mutations, mainly due to the absence of an effective repair system. Mutations in mtDNA are associated with endocrine, metabolic, neurodegenerative diseases, and even cancer. Mitochondrial gene therapy emerges as a promising strategy as it deeply focuses on the cause of mitochondrial disorder. The development of suitable mtDNA-based delivery systems to target and transfect mammalian mitochondria represents an exciting field of research, leading to progress in the challenging task of restoring mitochondria’s normal function.
1. Introduction
Mitochondria are small organelles present in eukaryotic cells that play a key role in maintaining cellular activity through intracellular signaling and energy production processes. These multifunctional organelles adapt their functioning depending on the cells in which they are present, having specific functions in different cell types [
4]. Mitochondria are the engines of cells, as they are responsible for about 90% of the energy that is produced in each cell. Through the mitochondrial oxidative phosphorylation system (OXPHOS), the oxidative phosphorylation of glucose occurs in the mitochondria, which gives rise to energy in the form of adenosine triphosphate (ATP) molecules [
5]. In addition to its energetic function, mitochondria are involved in a wide range of biological processes within the cell, namely in amino acid metabolism, protein synthesis, gluconeogenesis, fatty acid oxidation, generation of reactive oxygen species (ROS), ions and calcium homeostasis and initiation of apoptotic cascade [
6,
7,
8]. In addition, mitochondria play a fundamental role in oxidative stress situations, endoplasmic reticulum stress, and stress due to the lack of nutrients that are involved in the origin of DNA and RNA molecules and the processes of transcription correction [
9].
Mitochondria have their own DNA allowing their reproduction without the need for outside stimuli. These unique characteristics tend to prove the bacterial origin of this cell organelle. Despite having their own genome, many of the mitochondrial genes were passed to the nucleus, still maintaining a small number of genes that encode essential and exclusive proteins. Among these proteins expressed, only in the mitochondrial matrix, are the four enzymes of the OXPHOS complex [
10]. Mitochondria are formed by an outer and an inner membrane in which the inner membrane is folded and forms layers (cristae). They also have an intermembrane space, ribosomes, and genetic material. These organelles, with a size of around 1 micron, can be found in large numbers (1000 to 2000) in the cytoplasm of eukaryotic cells [
11].
The mitochondrial genome is made up of circular double-stranded DNA molecules, a characteristic indicating the bacterial origin of mitochondria [
12]. Mitochondria evolved from proteobacteria, and throughout this evolution, these organelles had significant changes in their genome, where there was a significant gain of new genes and the loss and transfer of others to the nuclear genome. This transition led to changes in the mitochondrial proteome and the development of additional roles in both the metabolism and biosynthetic pathways, forming a specialized organelle for ATP production [
13,
14].
Metabolic disorders resulting from changes that occur in genes regulating mitochondrial function are called primary mitochondrial diseases (PMDs) [
30]. PMDs are characterized by having different origins and depending on the affected gene can result in multiple metabolic disorders. These disturbances in mitochondrial function and structure will compromise its normal activity, namely the processes of oxidative phosphorylation, mitochondrial fission and fusion, and the process of ion transport across the mitochondrial membrane. Both mtDNA and nuclear DNA mutations can give rise to PMDs and cause mitochondria-associated metabolic changes [
31]. Due to the presence of mitochondria in almost every type of organ in the human body, PMDs rarely involve just a single tissue. PMDs can affect many organs and the age factor does not seem to have an influence [
32].
Mutations in mtDNA occur at a much higher frequency than mutations in nuclear DNA. These mutations result from errors during replication, and these errors often remain uncorrected. Mutations in mtDNA can also be transmitted by maternal mtDNA, arise from antecedent mutations in nuclear DNA, or result from environmental factors [
33]. One of the cases of environmental factors is stress which causes overexpression of reactive oxygen species (ROS). ROS are produced during the process of oxidative phosphorylation by the OXPHOS system, however, their production is at low levels and their presence is fundamental in physiological functions. Increased ROS levels can cause numerous mutations in mtDNA since they are produced within the mitochondria with high proximity to the genetic material [
34]. The fact that mtDNA lacks protective histones also makes it more susceptible to mutations caused by ROS [
35]. Elevated levels of ROS in situations where antioxidant enzymes are reduced can result in changes in both proteins and lipids and in the mtDNA itself, leading to the appearance of mitochondrial dysfunction. In extreme cases, mutations caused by ROS can alter the OXPHOS system and lead to the production of even more ROS, causing successive changes in mitochondrial metabolism and inducing a decrease in ATP production, loss of cell communication, rupture of the mitochondrial membrane, and consequent apoptosis [
36].
Other recurrent factors for mitochondrial deregulation are the mutations that occur simultaneously in the mitochondrial genome and in genes of nuclear origin that influence mitochondrial metabolism. These mutations, when they appear in genes encoding proteins essential for the synthesis of ATP molecules, lead to the degradation of cells due to lack of energy and, consequently, contribute to the appearance of diseases of mitochondrial origin in the various organs of the human body. These mutations can also interfere with the normal functioning of the OXPHOS system, alter tissue specificities of different organs and alter metabolite homeostasis [
37].
2. Nanotechnology in Mitochondrial Gene Therapy
2.1. Mitochondrial Gene Therapy
The development of nanotechnology has allowed the conception/emergence of new therapies, one of them being gene therapy. Gene therapy is based on the use of recombinant DNA techniques with functional genes to replace defective genes and consequently treat associated diseases [
85]. This type of therapy can be used to especially treat diseases originating in monogenetic changes or when mutations are well identified [
86]. Many of the dysfunctions that occur in mitochondria come from mutations in their genome, thus gene therapy emerges as a very promising approach to the treatment of mitochondrial diseases. Mitochondrial gene therapy arises from the need to find treatments for mitochondrial dysfunctions, as the solutions available on the market only serve to alleviate the symptoms and do not provide an effective cure [
87]. The main advantage of this approach, to conventional treatments, is that it focuses the problem on its origin replacing the mutated mitochondrial gene and restoring mitochondria function. In addition, mitochondrial gene therapy is a technique with reduced costs that can provide continuous treatment in time on target cells [
88].
Although most mitochondrial diseases in adulthood result from mtDNA mutations, alterations in nuclear genes that have a direct influence on mitochondrial metabolism also induce this type of disease, being the main cause of mitochondrial dysfunctions in children [
30,
89]. Thus, nucleus targeting has been explored to correct mitochondrial disorders. The application of indirect mitochondrial gene therapy aims at the translation of a protein originating from the genes transferred to the nucleus that is later imported into the mitochondria, reestablishing its normal function.
Gene therapy requires exogenous DNA to reach target cells. Physical, chemical, and biological methods have been explored in recent years to assess which approach is more convenient for each situation. Physical methods were the first approaches considered for the delivery of genetic material. These methods do not use carrier molecules for gene delivery. The advantages of these techniques are that transfection is not dependent on the ability of transporters to internalize cells and, therefore, there are no biocompatibility concerns related to the materials used in the conception of the delivery vectors. Despite this, physical methods can destroy the membrane of target cells as they are more invasive [
92]. In this regard, the most used techniques are microinjections and bioballistics. Microinjections consist of introducing genetic material with the help of a micropipette; however, this procedure requires a lot of experience and technique to avoid the bursting of the cell membrane. The bioballistic technique uses air pressure to project complexes of nucleic acids that are normally coated with metals [
93]. Other physical methods are emerging to overcome the disadvantages of these invasive techniques, which are the case of electroporation [
94], optoporation [
95], sonoporation [
96], and magnetoporation technique [
97]. These techniques aim to internalize the exogenous genetic material in cells through physical forces, being much less invasive and for many of them there is no contact with the cell membrane [
92].
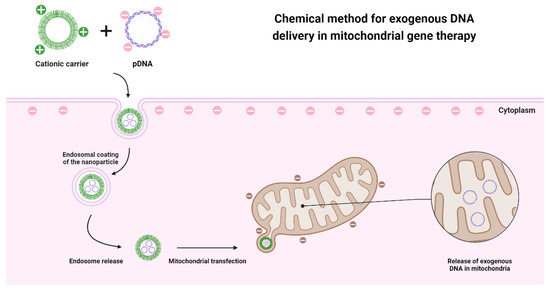
Although physical methods have some ability to internalize DNA into mitochondria, the success rate is limited. This fact instigates the development of other approaches that could bypass mitochondrial membranes. The most promising and most explored method is the chemical one since it meets the characteristics of mitochondrial and cell membranes. This method requires a cationic carrier that allows exogenous DNA to pass through the membranes, as both entities are negatively charged and would repel each other (Figure 3).
Figure 3. Representative illustration of the application of chemical methods in the delivery of genetic material to the mitochondria, based on the use of a cationic nanotransporter that through electrostatic interactions manages to penetrate both the cell membrane and the mitochondrial membranes.
One of the fundamental steps for this therapy is the choice of the nanocarrier that enables the delivery of therapeutic DNA. The characteristics to consider when choosing the vector are, mainly, its production on a large scale, its biocompatibility, and the ability to carry DNA. Since mitochondria have specific receptors and have a double membrane that is impermeable to hydrophilic molecules, there is a need to create new approaches and specific therapeutic vectors for mitochondrial gene therapy [
88,
100]. Due to the difficulty in targeting delivery systems to mitochondria, it is convenient to explore their receptors to facilitate their uptake by this organelle. It was found that mitochondrial proteins that are expressed in the cytosol enter the mitochondria through the translocase of the mitochondrial outer membrane (TOM) and mitochondrial inner membrane (TIM) allowing their recognition due to a sequence at the
N-terminus of these proteins [
101]. The presence of the mitochondrial targeting signal peptide (MTS) sequence allows preferential recognition of the TOM20—TOM22 receptor and thus translocation of proteins across mitochondrial membranes [
102]. Therefore, the MTS sequence has been used as a ligand in mitochondrial gene therapy, mediated by delivery systems for a targeted delivery [
103,
104].
2.2. Nanotechnology
Considered one of the most promising technologies of this century, nanotechnology allows the development of nanoscale materials for applications in the most diverse aspects of society using nanoscience as a theory. It allows the creation of technology at the nanometer scale which finds applications in areas such as engineering, electronics, physics, chemistry, biology, and medicine. Nanotechnology, by definition, deals with the design/development and application of materials with sizes between 1 and 100 nm. It also intends to tailor and optimize the physicochemical properties of these materials, since they can influence their interactions and functionalities [
105].
Inevitably, nanotechnology has been extensively explored in the field of oncology, where its assets have been explored to develop new treatment methodologies and to improve the effectiveness of drugs in conventional chemotherapy. The application of nanoparticles allowed the development of more targeted therapies for tumors, using functionalized molecules. These nanoparticles can be used as direct therapeutic agents or to serve as carriers of therapeutic biomolecules with anticancer activity [
108,
109]. Additionally, nanotechnology has been applied to perform diagnosis simultaneously with the therapeutic function. The principle of nanotheranostics is the development of a nanosystem to provide a desired therapeutic effect and, at the same time, allow the visualization of the tissue or cells targeted by this therapy [
110]. This technology can, for example, monitor the drug release and its biodistribution as well as evaluate at the same time the therapeutic effect of the drug delivered by the nanosystem. These nanoscale systems also ensure a targeted delivery to specific cells or tissues, using ligands that will be recognized by the receptors of the target cells, providing a more personalized and targeted therapy [
111].
Nanotechnology can also be used to identify biomarkers associated with mitochondria and explore them as a method of detecting mitochondrial disorders and early diagnosis. This technology can use mitochondrial biomarkers associated with specific mutations and thus combine them with gene therapy to develop new therapeutic strategies [
112]. Another approach is the application of mtDNA itself as a biomarker to identify possible changes and pathologies and to assess the body’s response to drug dosages, particularly in chemotherapy [
113]. The identified mitochondrial biomarkers can then be used in gene therapy as therapeutic targets of diseases and enable the intervention in processes such as mitophagy, post-transcriptional regulation, modification of mitochondria, and interactions of this organelle with other cellular organelles [
114].
2.3. Delivery Systems in Mitochondrial Gene Therapy
2.3.1. Polymers
Polymers are one of the most used materials to formulate nanocarriers. Their properties, such as easy manipulation of their structure and composition, ability to incorporate ligands, fast and economical production, and biocompatibility, make this type of material one of the most explored in gene therapy. Mitochondrial gene therapy is no exception, with several polymer-based delivery systems being developed that target mitochondria exclusively [
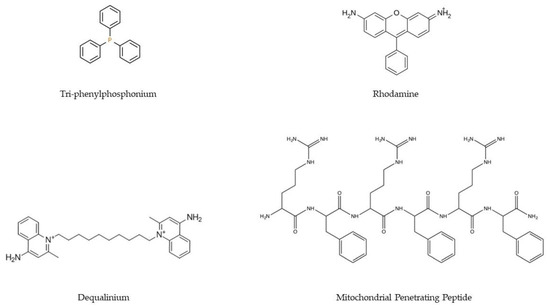
117]. Polymeric systems targeting mitochondria must overcome more barriers than systems that aim to solely cross the cell membrane. It is, therefore, imperative that these transporters are supplemented with ligands that allow mitochondria targeting. To this goal, the most commonly applied ligands are lipophilic cations (e.g., triphenylphosphonium, TPP/Dequalinium, DQA/Rhodamine), MTS/MPP (
Figure 4), and DNA and RNA aptamers. These ligands are intended to confer mitochondriotropic properties on polyplexes [
117,
118].
Figure 4. Chemical structures of the main ligands (TPP, rhodamine, DQA, and MPP) used in the development of delivery systems to confer targeting to the mitochondria.
2.3.2. Dendrimers
Dendrimers are highly branched polymer-based nanostructures, consisting of a core, branches, and surface groups [
125]. The structure and synthesis of dendrimers make this type of delivery system very dynamic and capable of being molded to the intended use. The core–shell structure that characterizes dendrimers allows branch points to be created in their synthesis, added to the vector nucleus, and the shell can be functionalized with ligands that confer specificity. In addition to this synthetic versatility, the fact that it has a core facilitates the encapsulation of molecules inside this type of nanocarrier [
126,
127].
2.3.3. Lipids
The use of lipids for the creation of gene-delivery nanosystems has been extensively explored for many years. This is due to their properties which allow the formation of a stable nanosystem due to the electrostatic interactions between nucleic acids and lipids but also based on a controlled size of the formed nanoparticles and the protection of the therapeutics inside the core of these transporters [
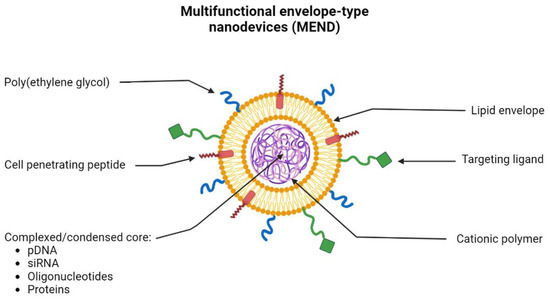
130]. In recent years, the use of multifunctional envelope-type nanodevices (MEND) has been widely explored. This type of lipid-based system allows the encapsulation of different types of molecules such as pDNA, RNA, oligonucleotides, and proteins. Due to the properties of lipids, they can be functionalized with multiple ligands (
Figure 6). MEND allows, for example, to add of peptides to its lipid envelope, improving the fusion and internalization of these systems in cells. Ligands can also be added to provide specific targeting to a certain organelle and PEG moieties to improve and prolong the circulation of lipid-based nanoparticles in the bloodstream [
131].
Figure 6. Schematic representation of the morphology and constitution of the multifunctional envelope-type nanodevice (MEND). MEND consists of a lipid envelope that is modified with cell-penetrating peptides (CPP), poly(ethylene glycol) (PEG), and ligands that provide specific targeting. Inside, MEND can encapsulate/condense various therapeutic materials such as DNA, RNA, proteins, etc.
Another type of lipid-based compound widely used for the delivery of nucleic acids to mitochondria is DQAsomes. DQAsomes are positively charged lipid vesicles that allow the encapsulation of nucleic acids and display an affinity for binding to the membrane of mitochondria [
100]. These complexes can escape from the endosomes without losing the encapsulated genetic material and carry the therapeutic payload next to the mitochondria or inside the mitochondrial matrix, depending on its composition [
136,
137].
2.3.4. Inorganic Nanoparticles
Rhodamine is a fluorescent compound with affinity to mitochondria, which facilitates the tracking of internalization into cells, and it has been applied as a probe for mitochondrial membrane potential [
143]. This property of rhodamine has been explored for the targeting and gene delivery to mitochondria, as described by Santos et al. [
144]. The researchers developed plasmid DNA-based rhodamine nanoparticles, using CaCl
2, Na
2CO
3, and cellulose, in a co-precipitation method, to formulate the nano-systems. The inorganic compounds were able to promote the encapsulation of three different plasmids and internalize them in several types of cell lines, also demonstrating the ability to target mitochondria, confirmed by confocal microscopy [
144]. The team of Costa formulated CaCO
3-pDNA-Rho123 nanoparticles using the same co-precipitation method [
145]. The vectors demonstrated the ability to encapsulate a plasmid containing the GFP gene, formulating stable nanoparticles of suitable size and surface charge for cell transfection. These delivery systems have demonstrated transfection capability into both normal and tumoral cells. Images obtained by two- and three-dimensional fluorescence confocal microscopy confirmed the targeting of these vectors to mitochondria in both fibroblasts and HeLa cells. This targeting was demonstrated by rhodamine fluorescence and by the expression of the GFP protein. To confirm the affinity of the systems for mitochondria, after cellular transfection, mitochondria were separated from the cytosolic fraction, verifying greater accumulation of nanoparticles in isolated mitochondria compared to the cytosol fraction, through quantification of rhodamine fluorescence [
145].
2.4. Strategies to Be Further Explored for Progress in Mitochondrial Gene Therapy
2.4.1. Dendrigraftpoly-L-lysines Based Delivery Systems
Biocompatible dendritic poly(L-lysine) (DGL) is a promising carrier for targeted drug/DNA delivery [
147]. DGL is becoming one of the most versatile nanoscale drug/DNA carriers due to its highly branched 3D architecture containing an initiator core, several inner layers composed of repeating units, and many outer amino groups [
148]. Compared with traditional nanocarriers, self-organized DGL nanoparticles are vastly superior in targeted therapy at the subcellular level due to their small size and their surface modifications (PEGylation and targeted ligands). In particular, DGL nanoparticles (DGL NPs) have a greater ability to facilitate intracellular internalization via endocytosis, and then in the endosomes, they act as proton-sponges to induce endo-lysosomal escape by osmotic swelling [
149]. These properties of DGL NPs make them attractive nanocarriers for the construction of targeted drug delivery systems to mitochondria.
2.4.2. ROS-Responsive PLGA-Based Delivery Systems
The ROS-responsive berberine PLGA-based nanocarriers (BPseP) were designed with the idea that tissue areas with high inflammation and high ROS concentration could irritate the cleavage of the mPEG–Se–Se–PLGA amphiphilic co-polymers and berberine will be released to produce higher ROS, which further facilitates the collapse of micelles [
151]. These specific ROS-responsive delivery systems were studied to develop a therapy for rheumatoid arthritis (RA), an autoimmune disease with no cure. Indeed, Fan and co-workers demonstrated that in RA fibroblasts the drug uptake was ten times higher compared to what happened in non-treated fibroblasts. The therapeutic effect obtained through mitochondrial targeting and drug release in the inflationary zone was shown by the inhibition of cell proliferation and elimination of lipogenesis, improving the therapeutic efficacy of AR [
151].
2.4.3. MITO-Porter-Based Systems
MITO-Porter is a liposome-based carrier that delivers macromolecules efficiently to the cytoplasm, as well as to mitochondria. In 2008, Yamada et al. coated the MITO-Porter surface with high-density octaarginine (R8) to deliver green fluorescence protein (GFP) to rat-liver mitochondria [
138]. Membrane fusion occurs by two extremely fusogenic lipid compositions: sphingomyelin (SM) and phosphatidic acid (PA) resulting in macropinocytosis instead of clathrin-mediated endocytosis, which allowed particles to enter the cell without being damaged. The MITO-Porter was further optimized by the S2 peptide (Dmt-d-Arg-FK-Dmt-d-Arg-FK-NH2) instead of the R8 showing a high mitochondrial targeting activity with less cellular toxicity [
155].
2.4.4. Upgraded TPP-Based Systems
A study carried out by Sharma and the team compared a dendrimer in which TPP molecules (TPP-D-Cy5) were coupled with the same dendrimer without TPP (D-Cy5). They showed that although these systems do not have a fully specific targeting for mitochondria, systems with TPP had more affinity for mitochondria. However, D-Cy5 dendrimers were also able to accumulate in mitochondria but in reduced numbers. The authors of this study also demonstrated that cells transfected with TPP-D-Cy5 showed improvement in levels and markers of oxidative stress [157]. Li and co-workers developed a micelle where they coupled TPP (PEG-AIE-TPP), a nanocarrier synthesized to be targeted to the mitochondria and used in the treatment of cancer due to the pH sensitivity [158]. These investigators demonstrated that these micelles selectively accumulated in mitochondria through the conjugation of a fluorogenic that produced an aggregation-induced emission (AIE) effect. Through confocal microscopy, it was possible to observe the superposition of this signal produced by PEG-AIE-TPP nanoparticles with the labeling of mitochondria. These micelles were used in MCF-7 tumor-bearing mice, where they were observed to preferentially accumulate in the tumor both in vivo and ex vivo [158].
Recent studies have revealed that the TPP+ moiety has detrimental effects on mitochondrial bioenergetics such as increasing proton leak and uncoupling mitochondrial oxidative phosphorylation (OXPHOS). Therefore, efforts were performed to identify TPP+ derivatives associated with mitochondrial uptake without OXPHOS decoupling to develop improved TPP+ derivatives for targeting cargos/carriers to mitochondria [161].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15020572