Biomarkers can provide critical information about cancer and many other diseases; therefore, developing analytical systems for recognising biomarkers is an essential direction in bioanalytical chemistry. Molecularly imprinted polymers (MIPs) have been applied in analytical systems to determine biomarkers. The most attractive way to replace natural biological recognition systems is based on applying molecularly imprinted polymers.
- molecularly imprinted polymer (MIP)
- conducting polymer (CP)
- electrochemical sensor
- cancer biosensors
- prostate cancer biomarker
- breast cancer biomarker
- epithelial ovarian cancerbiomarker
- hepatocellular carcinoma biomarker
- biomarker detection
- diagnostics
1. Introduction
Biomarkers can provide critical information about cancer and many other diseases; therefore, developing analytical systems for recognising biomarkers is an essential direction in bioanalytical chemistry [1], therefore, biosensor development technologies are evolving rapidly. Conducting polymers are frequently used to advance the analytical performance of sensors [2][3][4][5][6][7][8][9][10][11][12][13][14][15], particularly for the selectivity towards targeted analytes [15][8]. MIPs are used as artificial receptors to detect target molecules in sensors that act as smart electrochemical output devices. Preparation of the MIP involves several steps (Figure 1). Molecularly imprinted polymers (MIPs) are created by polymerising functional monomers on different types of electrode surfaces such as pencil graphite electrode [2], graphite electrode [3], boron-doped nanocrystalline diamond [4][5], platinum electrode [6][7], or the gold-coated quartz crystal microbalance [8][9] and surface plasmon resonance sensors disks [10] to form complementary sites for target molecules. After analytes are removed, they leave fitting imprinted cavities to recognize target molecules such as low molar mass molecules (e.g. theophylline [4][5], caffeine [8], histamine [11], uric acid [9][12], tryptophan [3], etc.), or large molar mass substances such as proteins [6][7][13] or DNA [2], or even living entities such as bacteria [14]. The high specificity, selectivity, sensitivity for target molecules, and detection ability of MIP-based electrochemical biosensors open vast possibilities for disease diagnostics.
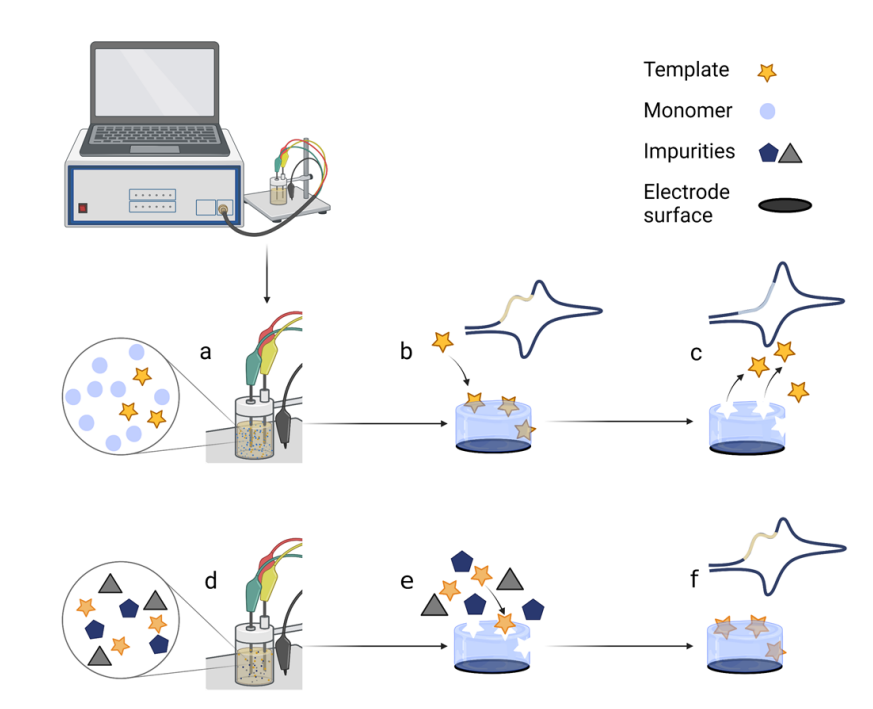
Figure 1. Scheme of the electrochemical polymerisation of the MIP directly on the electrode and the application of the MIP-based electrochemical biosensor: (a) polymerisation mixture preparation; (b) polymerisation of the polymer with template imprints layer on the electrode; (c) extraction of the template from the MIP; (d) application of the MIP-based sensor; (e) competitive interaction of target molecules on the MIP in presence of interferents; (f) detection of target molecules. All drawings were designed using Biorender.com, accessed on 26 December 2022.
2. Biomarker of Prostate Cancer—PSA
3. Biomarkers of Breast Cancer
3.1. CA15-3
3.2. HER-2
4. Biomarker of Epithelial Ovarian Cancer—CA-125
5. Biomarker of Hepatocellular Carcinoma—AFP
| Polymer | Electrode | Detection Method | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|
| Myo | |||||
| Poly(N, N’-methylenebisacrylamide-acrylamide) | AuSPE | EIS | 1–20,000 ng/mL | 0.83 ng/mL | [17] |
| PSA | |||||
| Poly(N, N’-methylenebisacrylamide-acrylamide) | AuSPE | EIS | 0.01–100 ng/mL | 5.4 pg/mL | [17] |
| Ppy | AuSPE | DPV | 0.01–4 ng/mL | 2.0 pg/mL | [19] |
| Poly(toluidine blue) | AuE | DPV | 1–60 μg/L | 1 μg/L | [18] |
| PDA | AuE | DPV | 0.100–100 ng/mL | 1 pg/mL | [20] |
| PDA | AuE | MOSFET | 0.1 pg/mL–1 ng/mL | 0.1 pg/mL | [22] |
| CA15-3 | |||||
| Poly(O-aminophenol) | AuSPE | DPV, EIS | 5–50 U/mL | 1.5 U/mL | [27] |
| Poly(toluidine blue) | AuSPE | DPV | 0.10–100 U/mL | 0.10 U/mL | [25] |
| Ppy | FTO-glass | EMF | 1.44 to 13.2 U/mL | 1.072 U/mL | [26] |
| CA-125 | |||||
| Ppy | AuSPE | SWV | 0.01–500 U/mL | 0.01 U/mL | [36] |
| AFP | |||||
| PDA | GCE | DPV | 0.001–800 ng/mL | 0.8138 pg/mL | [42] |
| Ppy | FTO | CV, EIS | 10–104 pg/mL | 3.3 pg/mL | [43] |
| HER-2 | |||||
| Poly(3,4-ethylenedioxythiophene) | LSG | SWV, EIS | - | 0.43 ng/mL | [31] |
| Polyphenol | AuSPE | EIS, DPV | 10–70 ng/mL | 1.6 ng/mL | [32] |
6. Detection of Small Molecule Cancer Biomarkers: 5-HIAA and Neopterin
7. Conclusions
The overviewed studies have shown that MIPs can be successfully applied in developing biosensors for cancer biomarkers. MIP-based biosensors with conducting polymers gave better LODs for PSA, CA15-3, HER-2, CA-125, and neopterin biomarkers determination than non-conducting polymers. In conclusion, MIP-based electrochemical biosensors were used to detect various cancer biomarkers such as proteins (PSA, Myo, CA15-3, HER-2, CA-125) or small molecules (5-HIAA, neopterin). Therefore, it could be an alternative to expensive and time-consuming laboratory tests. In the reviewed articles, gold was mainly chosen for electrodes. Other types of electrodes, such as GCE and LSG electrodes, were combined with gold nanoparticles. SPE became a common choice recently due to its convenient clinical use. Since the determination of multiple biomarkers is usually required for cancer detection, it would be advantageous to develop biosensors capable of simultaneously detecting several biomarkers. Overall, these biosensors offer a simple, sensitive, and low-cost analysis required for the early diagnosis of cancer in comparison with routine laboratory tests. Many new technical developments of MIP-based biosensors are currently under intense study to help achieve this goal at the multidisciplinary interface of chemistry, biology, and material science. As discussed in this review, MIP-based biosensors have many diagnostic possibilities for detecting various cancer biomarkers, such as proteins (PSA, Myo, CA15-3, HER-2, CA-125) or small-weight molecules (5-HIAA, neopterin) due to their robustness, sensitivity, and inexpensive analysis.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24044105
References
- Pilvenyte G.; Ratautaite V.; Boguzaite R.; Ramanavicius A.; Viter R.; Ramanavicius S.; Molecularly Imprinted Polymers for the Determination of Cancer Biomarkers. Int. J. Mol. Sci. 2023, 24, 4105, https://doi.org/10.3390/ ijms24044105.
- V. Ratautaite, S.N. Topkaya, L. Mikoliunaite, M. Ozsoz, Y. Oztekin, A. Ramanaviciene, A. Ramanavicius; Molecularly imprinted polypyrrole for DNA determination. Electroanalysis 2013, 25 (5), 1169–1177, https://doi.org/ 10.1002/elan.201300063.
- V. Ratautaite, E. Brazys, A. Ramanaviciene, A. Ramanavicius; Electrochemical sensors based on l-tryptophan molecularly imprinted polypyrrole and polyaniline. J. Electroanal. Chem. 2022, 917, 116389, https://doi.org/10.1016/j.jelechem.2022.116389.
- I. Baleviciute, V. Ratautaite, A. Ramanaviciene, Z. Balevicius, J. Broeders, D. Croux, M. McDonald, F. Vahidpour, R. Thoelen, W.D. Ceuninck, K. Haenen, M. Nesladek, A. Reza, A. Ramanavicius; Evaluation of theophylline imprinted polypyrrole film. Synth. Met. 2015, 209, 206–211, https://doi.org/10.1016/j.synthmet.2015.07.021.
- V. Ratautaite, S.D. Janssens, K. Haenen, M. Nesl´ adek, A. Ramanaviciene, I. Baleviciute, A. Ramanavicius; Molecularly imprinted polypyrrole based impedimentric sensor for theophylline determination. Electrochim. Acta 2014, 130 (0), 361–367, https://doi.org/10.1016/j.electacta.2014.03.035.
- V. Ratautaite, R. Boguzaite, E. Brazys, D. Plausinaitis, S. Ramanavicius, U. Samukaite-Bubniene, M. Bechelany, A. Ramanavicius; Evaluation of the interaction between SARS-CoV-2 spike glycoproteins and the molecularly imprinted polypyrrole. Talanta 2023, 253, 123981, https://doi.org/10.1016/j.talanta.2022.123981.
- V. Ratautaite, R. Boguzaite, E. Brazys, A. Ramanaviciene, E. Ciplys, M. Juozapaitis, R. Slibinskas, M. Bechelany, A. Ramanavicius; Molecularly imprinted polypyrrole based sensor for the detection of SARS-CoV-2 spike glycoprotein. Electrochim. Acta 2022, 403, 139581, https://doi.org/10.1016/j.electacta.2021.139581.
- V. Ratautaite, D. Plausinaitis, I. Baleviciute, L. Mikoliunaite, A. Ramanaviciene, A. Ramanavicius; Characterization of caffeine-imprinted polypyrrole by a quartz crystal microbalance and electrochemical impedance spectroscopy. Sens. Actuators B-Chem. 2015, 212, 63–71, https://doi.org/10.1016/j.snb.2015.01.109.
- D. Plausinaitis, L. Sinkevicius, U. Samukaite-Bubniene, V. Ratautaite, A. Ramanavicius; Evaluation of electrochemical quartz crystal microbalance based sensor modified by uric acid-imprinted polypyrrole. Talanta 2020, 220, 21414, https://doi.org/10.1016/j.talanta.2020.121414.
- D. Balciunas, D. Plausinaitis, V. Ratautaite, A. Ramanaviciene, A. Ramanavicius; Towards electrochemical surface plasmon resonance sensor based on the molecularly imprinted polypyrrole for glyphosate sensing. Talanta 2022, 241, 123252, https://doi.org/10.1016/j.talanta.2022.123252.
- V. Ratautaite, M. Nesladek, A. Ramanaviciene, I. Baleviciute, A. Ramanavicius; Evaluation of histamine imprinted polypyrrole deposited on boron doped nanocrystalline diamond. Electroanalysis 2014, 26, 2458–2464, https://doi.org/10.1002/elan.201400294.
- V. Ratautaite, U. Samukaite-Bubniene, D. Plausinaitis, R. Boguzaite, D. Balciunas, A. Ramanaviciene, G. Neunert, A. Ramanavicius; Molecular imprinting technology for determination of uric acid. Int. J. Mol. Sci. 2021, 22 (9), 5032, https://doi.org/10.3390/ijms22095032.
- A. Ramanaviciene, A. Ramanavicius; Molecularly imprinted polypyrrole-based synthetic receptor for direct detection of bovine leukemia virus glycoproteins. Biosens. Bioelectron. 2004, 20 (6), 1076–1082, https://doi.org/10.1016/j.bios.2004.05.014.
- Liustrovaite V, Pogorielov M, Boguzaite R, Ratautaite V, Ramanaviciene A, Pilvenyte G, Holubnycha V, Korniienko V, Diedkova K, Viter R, Ramanavicius A.; Towards Electrochemical Sensor Based on Molecularly Imprinted Polypyrrole for the Detection of Bacteria—Listeria monocytogenes. Polymers 2023, 15(7), 1597, https://doi.org/10.3390/polym15071597.
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A.; Advances in Molecularly Imprinted Polymers Based Affinity Sensors. Polymers 2021, 13, 974, https://doi.org/10.3390/polym13060974.
- Sattarahmady, N.; Rahi, A.; Heli, H.; A signal-on built in-marker electrochemical aptasensor for human prostate-specific antigen based on a hairbrush-like gold nanostructure. Sci. Rep. 2017, 7, 11238, https://doi.org/10.1038/s41598-017-11680-5.
- Karami, P.; Bagheri, H.; Johari-Ahar, M.; Khoshsafar, H.; Arduini, F.; Afkhami, A.; Dual-modality impedimetric immunosensor for early detection of prostate-specific antigen and myoglobin markers based on antibody-molecularly imprinted polymer.. Talanta 2019, 202, 111–122, https://doi.org/10.1016/j.talanta.2019.04.061.
- Abbasy, L.; Mohammadzadeh, A.; Hasanzadeh, M.; Razmi, N.; Development of a reliable bioanalytical method based on prostate specific antigen trapping on the cavity of molecular imprinted polymer towards sensing of PSA using binding affinity of PSA-MIP receptor: A novel biosensor.. Pharm. Biomed. Anal. 2020, 188, 113447, https://doi.org/10.1016/j.jpba.2020.113447.
- Yazdani, Z.; Yadegari, H.; Heli, H.; A molecularly imprinted electrochemical nanobiosensor for prostate specific antigen determination.. Anal. Biochem. 2019, 566, 116–125, https://doi.org/10.1016/j.ab.2018.11.020.
- Jolly, P.; Tamboli, V.; Harniman, R.L.; Estrela, P.; Allender, C.J.; Bowen, J.L.; Aptamer—MIP hybrid receptor for highly sensitive electrochemical detection of prostate specific antigen.. Biosens. Bioelectron. 2016, 75, 188–195. , .
- Jin, Z.; Yang, L.; Shi, S.; Wang, T.; Duan, G.; Liu, X.; Li, Y.; Flexible Polydopamine Bioelectronics. Adv. Funct. Mater 2021, 31, 2103391, https://doi.org/10.1002/adfm.202103391.
- Tamboli, V.K.; Bhalla, N.; Jolly, P.; Bowen, C.R.; Taylor, J.T.; Bowen, J.L.; Allender, C.J.; Estrela, P.; Hybrid Synthetic Receptors on MOSFET Devices for Detection of Prostate Specific Antigen in Human Plasma. Anal. Chem. 2016, 88, 11486–11490, https://doi.org/10.1021/acs.analchem.6b02619.
- Diagnostic Tests. Tumor Markers . National Cancer Institute. SEER Training Modules. Retrieved 2023-4-16
- Cancer Antigen 15-3 (CA15-3) Test . Canadian Cancer Society. Retrieved 2023-4-16
- Ribeiro, J.A.; Pereira, C.M.; Silva, A.F.; Sales, M.G.F.; Disposable electrochemical detection of breast cancer tumour marker CA 15-3 using poly(Toluidine Blue) as imprinted polymer receptor. Biosens. Bioelectron. 2018, 109, 246-254, https://doi.org/10.1016/j.bios.2018.03.011.
- Santos, A.R.T.; Moreira, F.T.C.; Helguero, L.A.; Sales, M.G.F.; Antibody Biomimetic Material Made of Pyrrole for CA 15-3 and Its Application as Sensing Material in Ion-Selective Electrodes for Potentiometric Detection. Biosensors 2018, 8, 8, https://doi.org/10.3390/bios8010008.
- Pacheco, J.G.; Silva, M.S.V.; Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C.; Molecularly imprinted electrochemical sensor for the point-of-care detection of a breast cancer biomarker (CA 15-3). Sens. Actuat. B-Chem. 2018, 256, 905–912, https://doi.org/10.1016/j.snb.2017.10.027.
- Krishnamurti, U.; Silverman, J.F.; HER2 in Breast Cancer: A Review and Update. Adv. Anat. Pathol. 2014, 21, 100–107, DOI: 10.1097/PAP.0000000000000015.
- Cole, K.D.; He, H.-J.; Wang, L.; Breast cancer biomarker measurements and standards. Proteom.–Clin. Appl. 2013, 7, 17–29, https://doi.org/10.1002/prca.201200075.
- Shamshirian, A.; Aref, A.R.; Yip, G.W.; Ebrahimi Warkiani, M.; Heydari, K.; Razavi Bazaz, S.; Hamzehgardeshi, Z.; Shamshirian, D.; Moosazadeh, M.; Alizadeh-Navaei, R.; et al. Diagnostic value of serum HER2 levels in breast cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 1049, https://doi.org/10.1186/s12885-020-07545-2.
- Lahcen, A.A.; Rauf, S.; Aljedaibi, A.; de Oliveira Filho, J.I.; Beduk, T.; Mani, V.; Alshareef, H.N.; Salama, K.N.; Laser-scribed graphene sensor based on gold nanostructures and molecularly imprinted polymers: Application for Her-2 cancer biomarker detection. Sens. Actuat. B-Chem. 2021, 347, 130556, https://doi.org/10.1016/j.snb.2021.130556.
- Pacheco, J.G.; Rebelo, P.; Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C.; Breast cancer biomarker (HER2-ECD) detection using a molecularly imprinted electrochemical sensor. Sens. Actuat. B-Chem. 2018, 273, 1008–1014, https://doi.org/10.1016/j.snb.2018.06.113.
- Ovarian Cancer: Recognition and Initial Management . National Institute for Health and Care Excellence. Retrieved 2023-4-16
- Zhao, L.; Ma, Z.; Facile synthesis of polyaniline-polythionine redox hydrogel: Conductive, antifouling and enzyme-linked material for ultrasensitive label-free amperometric immunosensor toward carcinoma antigen-125. Anal. Chim. Acta 2018, 997, 60–66, https://doi.org/10.1016/j.aca.2017.10.017.
- Büyüktiryaki, S.; Say, R.; Denizli, A.; Ersöz, A.; Phosphoserine imprinted nanosensor for detection of Cancer Antigen 125. Talanta 2017, 167, 172–180, https://doi.org/10.1016/j.talanta.2017.01.093.
- Rebelo, T.S.C.R.; Costa, R.; Brandão, A.T.S.C.; Silva, A.F.; Sales, M.G.F.; Pereira, C.M.; Molecularly imprinted polymer SPE sensor for analysis of CA-125 on serum. Anal. Chim. Acta 2019, 1082, 126–135, https://doi.org/10.1016/j.aca.2019.07.050.
- Viswanathan, S.; Rani, C.; Ribeiro, S.; Delerue-Matos, C.; Molecular imprinted nanoelectrodes for ultra sensitive detection of ovarian cancer marker. Biosens. Bioelectron. 2012, 33, 179–183, https://doi.org/10.1016/j.bios.2011.12.049.
- Bahari, D.; Babamiri, B.; Salimi, A.; Ultrasensitive molecularly imprinted fluorescence sensor for simultaneous determination of CA125 and CA15-3 in human serum and OVCAR-3 and MCF-7 cells lines using Cd and Ni nanoclusters as new emitters. Anal. Bioanal. Chem. 2021, 413, 4049–4061, https://doi.org/10.1007/s00216-021-03362-z.
- Trevisani, F.; Garuti, F.; Neri, A.; Alpha-fetoprotein for Diagnosis, Prognosis, and Transplant Selection. Semin. Liver Dis. 2019, 39, 163–177, DOI: 10.1055/s-0039-1677768.
- ABIM (American Board Of Internal Medicine) Laboratory Test Reference Ranges—January 2022 . American Board of Internal Medicine. Retrieved 2023-4-16
- Zhang, J.; Chen, G.; Zhang, P.; Zhang, J.; Li, X.; Gan, D.n.; Cao, X.; Han, M.; Du, H.; Ye, Y.A.; et al. The threshold of alpha-fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0228857, https://doi.org/10.1371/journal.pone.0228857.
- Lai, Y.; Zhang, C.; Deng, Y.; Yang, G.; Li, S.; Tang, C.; He, N.; A novel α-fetoprotein-MIP immunosensor based on AuNPs/PTh modified glass carbon electrode. Chin. Chem. Lett. 2019, 30, 160–162, https://doi.org/10.1016/j.cclet.2018.07.011.
- Taheri, N.; Khoshsafar, H.; Ghanei, M.; Ghazvini, A.; Bagheri, H.; Dual-template rectangular nanotube molecularly imprinted polypyrrole for label-free impedimetric sensing of AFP and CEA as lung cancer biomarkers. Talanta 2022, 239, 123146, https://doi.org/10.1016/j.talanta.2021.123146.
- Li, Y.; Bober, P.; Trchová, M.; Stejskal, J.; Polypyrrole prepared in the presence of methyl orange and ethyl orange: Nanotubes versus globules in conductivity enhancement. J. Mater. Chem. C 2017, 5, 4236–4245, DOI https://doi.org/10.1039/C7TC00206H.
- Ewang-Emukowhate, M.; Nair, D.; Caplin, M.; The role of 5-hydroxyindoleacetic acid in neuroendocrine tumors: The journey so far. Int. J. Endocr. Oncol. 2019, 6, IJE17, https://doi.org/10.2217/ije-2019-0001.
- Moncer, F.; Adhoum, N.; Catak, D.; Monser, L.; Electrochemical sensor based on MIP for highly sensitive detection of 5-hydroxyindole-3-acetic acid carcinoid cancer biomarker in human biological fluids. Anal. Chim. Acta 2021, 1181, 338925, https://doi.org/10.1016/j.aca.2021.338925.
- Turco, A.; Corvaglia, S.; Mazzotta, E.; Electrochemical sensor for sulfadimethoxine based on molecularly imprinted polypyrrole: Study of imprinting parameters. Biosens. Bioelectron. 2015, 63, 240–247, https://doi.org/10.1016/j.bios.2014.07.045.
- Del Sole, R.; Scardino, A.; Lazzoi, M.R.; Mergola, L.; Scorrano, S.; Vasapollo, G.; A molecularly imprinted polymer for the determination of neopterin. Microchim. Acta 2013, 180, 1401–1409, https://doi.org/10.1007/s00604-013-0982-y.
- Robertson, J.; Gostner, J.M.; Nilsson, S.; Andersson, L.-M.; Fuchs, D.; Gisslen, M.; Serum neopterin levels in relation to mild and severe COVID-19. BMC Infect. Dis. 2020, 20, 942, https://doi.org/10.1186/s12879-020-05671-7.
- Berdowska, A.; Zwirska-Korczala, K.; Neopterin measurement in clinical diagnosis. J. Clin. Pharm. Ther. 2001, 26, 319–329, https://doi.org/10.1046/j.1365-2710.2001.00358.x.
- Eisenhut, M.; Neopterin in Diagnosis and Monitoring of Infectious Diseases. J. Biomark. 2013, 2013, 196432, https://doi.org/10.1155/2013/196432.
- Sharma, P.S.; Wojnarowicz, A.; Sosnowska, M.; Benincori, T.; Noworyta, K.; D’Souza, F.; Kutner, W.; Potentiometric chemosensor for neopterin, a cancer biomarker, using an electrochemically synthesized molecularly imprinted polymer as the recognition unit. Biosens. Bioelectron. 2016, 77, 565–572, https://doi.org/10.1016/j.bios.2015.10.013.
