The Draize eye irritation test was developed by the Food and Drug Administration (FDA) to assess the potential ocular toxicity of products, including cosmetics, insecticides, hair products, and sunscreens that were likely to come in contact with the eye during routine usage by the typical consumer [
5]. The test entails the exposure of one eye from each of three to six rabbits to a dosage of 0.1 mL or 0.1 g of the liquid or solid substance being studied [
6]. The focus of instillation is the lower conjunctival cul-de-sac of the rabbit eye [
7]. Effects on the conjunctiva, cornea, and iris, ranging from slight, reversible irritation to severe, irreversible irritation, and vision loss are observed and recorded based on a subjective scoring system [
8]. However, the “score” assigned to a chemical would be mainly associated with the degree of corneal injury and opacity present (80 points), with conjunctival irritation (20 points) and inflammation of the iris (10 points) being measured with lesser value on the overall “Maximum Average Score” determined from the average of the scores from each rabbit [
7]. Observations of eye irritation take place at specific intervals: 1, 24, 48, and 72 h, and 7 days after applications [
9].
The EpiOcular™ Eye Irritation Test (EIT), an in vitro 3D epithelial model, is commercially available from the MatTek Corporation. The EIT relies upon normal (non-transformed) human cells grown to form a stratified, squamous epithelium [
18,
19]. After a substance is applied to the model, the percent viability of the cell culture is commonly determined using an assay, often the 3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide (MTT) assay to test for cytotoxicity, where the MTT is reduced to formazan crystals by the mitochondria of the living cells. A highly cytotoxic irritant results in a loss of viability of the culture to 60.0% or less, whereas a viability in excess of 60% relative to a negative control suggests that the chemical is a non-irritant [
16,
20]. Others have used another viability assay, the lactate dehydrogenase (LDH) leakage assay to evaluate toxicity of chemicals. It is based on the release of the cytosolic LDH enzyme into extracellular medium by dead cells where its activity can be measured [
21]. ET50 values can be measured with MTT or LDH viability assays to determine relative cytotoxicity via comparisons with in vivo animal data [
22,
23]. These MTT and LDH cytotoxicity tests are indicators of reductions in cell viability. A greater speed and depth of injury or decline in cell viability from a substance denotes greater cytotoxicity [
15,
24]. Cytotoxicity corresponds to the ocular irritancy of the substance.
3. Pesticide Exposure
3.1. Pesticide Overview
Pesticides are potent environmental pollutants that are especially relevant to workers in the agricultural industry, exterminators, and pesticide manufacturers [
111]. Approximately 866 million workers are employed in agriculture worldwide representing about 20% of the world’s wage-earning labor force, making occupational exposure to pesticides a pressing global health concern [
112,
113]. Pesticide use has increased steadily, and exposure is a health concern for the general population since phenomena such as pesticide drift or the presence of residues in food or drinking water can have deleterious health consequences [
114,
115]. The reporting of pesticide exposure-related health concerns is complicated by the varying levels of toxicity of different agro-chemicals, as well as the variability in exposure level and route of exposure (ingestion, inhalation, skin, or mucous membrane absorption) [
116].
Pesticides, categorized as insecticides, herbicides, and fungicides, are often composed of organophosphates, organochlorines, and carbamate compounds [
117,
118,
119,
120]. These classes of compounds interact with several cellular receptors and interfere with normal bodily function.
The health concerns related to pesticide exposure have been extensively documented, and chronic exposure to toxic pesticides has been linked to increased risk of cancer, dermatoses, and genotoxic, neurotoxic, and respiratory consequences [
121,
122,
123]. Pesticide application leads to high levels of ocular exposure to toxic chemicals [
124]. Pesticides can easily make their way into the eye from accidental splashing or by rubbing the eye with contaminated hands or cloths or by absorption from the air [
125,
126]. While exposure to pesticides is common, the impact of the ocular route of exposure and its consequences is poorly understood. Unfortunately, there is a gap in the medical literature regarding the effects of pesticides, especially pesticides of different classes, on the ocular surface.
3.2. Herbicides and Insecticides
The herbicide paraquat, an organochlorine dipyridylium quaternary ammonium salt, is used frequently in agricultural fields and is known to be toxic to the ocular surface. Paraquat has been banned in European Union since 2007. Its toxicity is believed to relate to paraquat recycling in redox metabolism. Paraquat is an easily reducible organic cation, which interacts favorably with the reductive agent NADPH [
127]. NADPH is a cellular electron carrier involved in many bio-reductive pathways for cellular metabolism and easily donates an electron to paraquat to become NADP+. This causes disruptions in cellular metabolism, as it depletes the NADPH pool of the cell and interrupts metabolic homeostasis. The depletion of NADPH also causes the accumulation of oxygen free radicals such as superoxide since these species are reduced by NADPH as a cytoprotective measure. The generation of free radicals causes tissue damage at the ocular surface due to the highly reactive nature of free radicals, which steal electrons from key biological molecules. On the ocular surface, a common result of free radical damage is conjunctivalization of the cornea with vascular pannus [
127]. Severe injury may result in a chronically disordered ocular surface, manifesting in symptoms such as dryness, punctal stenosis, symblepharon, ankyloblpharon, forniceal shortening, entropion, and trichiasis [
128,
129]. Early appropriate treatment by flushing thoroughly with water may avoid highest levels of injury and minimize damage to minor corneal opacity and pannus as the main complications [
130]. Paraquat-containing pesticide mixtures such as preeglox-L, which also contains diquat and surfactants, have also been linked to corneal epithelium deterioration [
131].
Many herbicides contain the active ingredient glyphosate, an organophosphate compound that has toxic effects on several bodily systems. Organophosphates inhibit acetylcholinesterase (AChE), a key enzyme in the nervous system, by phosphorylating a serine hydroxyl group of its active site [
132,
133]. The inhibition of AChE by pesticides is known to cause eyelid muscle twitching, eye pain, and miosis [
132,
134]. Glyphosate has been shown to cause conjunctival irritation and superficial corneal injury, especially in cases where eye irrigation is delayed. [
135,
136].
Organophosphate exposure has also been linked to decreased glutathione content and increased levels of oxidative stress as measured by malondialdehyde levels in mouse eye and brain tissue upon exposure to the insecticide chlorpyrifos [
137,
138,
139]. Cellular disruption via organophosphate pesticide exposure may result from inhibition of antioxidant enzymes such as superoxide dismutase and catalase, as well as an increase in inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β [
140,
141,
142,
143,
144].
Flubendamide is a newer synthetic phthalic acid diamide insecticide with low immediate toxicity to humans [
145]. The effects of flubendiamide on the ocular surface were studied in non-target
Drosophila melanogaster to evaluate cross-reactivity in species at which the insecticide is not directed. It was found that flubendiamide altered the compound eye architecture and bristle pattern orientation in four generations of non-target
D. melanogaster at doses consistent with those administered in fields in India [
146,
147]. The irritative nature of flubendiamide is further explored in a report published by the Food Safety Commission of Japan, as the insecticide was linked to ocular inflammation in rats [
148].
3.3. Fungicides
Mancozeb, a manganese/zinc ethylene-bis-dithiocarbamate fungicide, inhibits enzyme activity in fungi by complexing with enzymes containing sulfhydryl groups including those that participate in generation of ATP. This carbamate pesticide has been shown to cause toxic epidermal necrolysis and ocular lesions in cases of human exposure [
149]. Carbamate pesticides, like organophosphate pesticides, are known to affect the AChE enzyme in human cells. Carbamates cause the carbamylation of AChE in neuronal synapses and neuromuscular junctions, and whereas organophosphates bind irreversibly to AChE, carbamates bind reversibly to the enzyme [
150].
A study conducted at a seed supply warehouse in Japan identified n-butyl isocyanate, a hydrolyzed product of the fungicide benomyl as the cause for ocular irritation among several workers [
151]. This finding has significant implications on regulatory measures for commercially used pesticides, as the safety of not only the pesticide must be taken into account but also the products of its degradation.
4. Workplace Ocular Injuries
4.1. Overview
The workplace is a common site of ocular injuries, as approximately 2000 U.S. workers experience job-related eye injuries requiring medical treatment each day [
152,
153]. These injuries can be divided into three broad categories: striking or scraping, penetrating, and chemical and thermal burns [
154,
155,
156]. Striking or scraping constitutes a common type of ocular injury, and involves the ejection of small particles such as dust, wood chips, or cement chips into the ocular surface, as well as larger objects that result in blunt trauma to the eye [
157]. Penetration occurs when objects such as nails, staples, or slivers of wood or metal move through the surface of the eye and potentially result in the permanent loss of vision [
158,
159]. Chemical and thermal burns to the eye are frequently caused by industrial chemicals and cleaning products, and welding processes respectively [
154]. A cross-sectional retrospective study used de-identified data from a large-scale employer survey of individuals reported to have ocular workplace injuries in the United States between 2011 and 2018 showed the highest likelihood of this type of injury in those employed in: fishing, farming and forestry; construction; and production industries [
160].
4.2. Foreign Object Injuries
In the fishing industry and in sports fishing, injury can occur when fishing hooks, lures, rod tips, or lines accidentally strike the eye [
161,
162,
163,
164]. Any eye structure may be involved with damage ranging from corneal abrasion to penetrating injury to globe rupture. Lenses, particularly wraparound lenses can protect the eye during fishing.
Wood injuries may occur in forestry workers, wood workers, and gardeners [
165]. Infections of bacterial or fungal origin are a significant risk, especially if the wood fragment is not removed promptly [
166,
167]. The high infection rate is attributed to the pores on the wood surface and the characteristics of organic and vegetative matter, which provide bacterial growth medium [
168]. The infection may manifest as orbital cellulitis, abscess formation, and even intracranial infection. Detection of wood in the eye is challenging because it is carbon-containing and not visible on conventional x-ray may not image well on CT or MRI [
169,
170]. If the chip is small and on the surface, it may be flushed with eyewash; however, deeper penetration shards may require surgical intervention and antibiotic treatment [
171].
Metal workers are particularly susceptible to dry eye according to a study by Ai et al. [
172]. They attribute the vulnerability of metal workers to dry eye disease to their exposure to dust and chemicals. In a cross-sectional study of welders in Turkey, exposure to cadmium and lead were correlated with dry eye disease [
173]. Chen et al. also found lead exposure and presence of lead in tears to be associated with dry eye disease [
174].
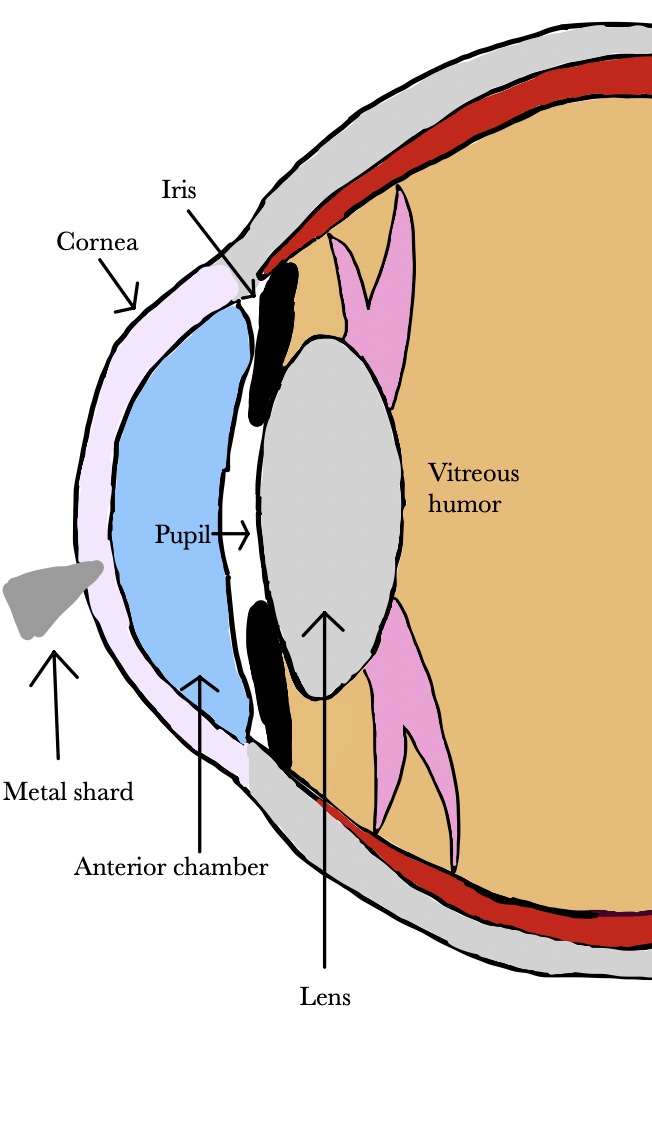
Metallic foreign bodies can enter the eye during use of hammer and nail, nail gun, or stapler (Figure 1) [
175,
176,
177,
178]. Metallic foreign body removal is key in order to avoid consequences such as infection, swelling, inflammation, astigmatism, and opacification of the cornea [
179]. Release of iron or copper from a retained foreign body in the eye can lead to cataracts, glaucoma, and pigment changes on the retina [
180,
181,
182].
4.3. Chemical Injuries
Cleaning products used around the home and office are often formulated with chemicals that can damage the eye. Chemical burns to the eye can come from acids, alkalis, or alcohol [
183]. Acids cause protein coagulation, which somewhat limits damage by forming a self-containing barrier while alkalis are lipophilic, cause saponification and penetrate more deeply into tissue, leading to extensive and severe damage to the cornea [
184,
185]. Alkali burns can result in loss of limbal epithelial stem cells that are essential for regeneration of corneal epithelium [
186].
In the United States, bleaches, categorized as alkali, accounted for more than 25% of ocular exposures reported to poison control centers between January 2000 and December 2016 [
187]. Bleach can cause burning sensation, tearing, photophobia, and conjunctival abrasions [
188,
189,
190].
Hydrofluoric acid is a highly reactive compound used in industry and some cleaning and rust-removing products. It can cause burns, tearing, conjunctivitis, and corneal ulcers and opacification [
191,
192].
Exposure of the eye to ethanol, which is often used as a disinfectant, can damage corneal epithelial and stromal cells, and cause inflammation and proinflammatory cytokine release [
193,
194].
4.4. Preventing Damage from Chemicals and Foreign Bodies
Particles in the eye and chemical eye burns require immediate flushing and therefore access to water or other rinsing solutions in the workplace is essential [
195]. Most occupational eye injuries are potentially preventable [
196]. Eye protection needs to fully cover the eyes [
197]. There are multiple forms of appropriate eye protection, some of which include goggles, face shields, and full-face respirators that reduce the likelihood of work-related eye injuries [
191,
198,
199,
200]. Indirectly vented goggles that fit from the corners of the eye across the brow provide effective protection from splashes, sprays, and respiratory droplets that may be encountered in the workplace [
156]. Although goggles are viable in shielding the eyes from irritants, other parts of the face are neglected by goggles and thus remain vulnerable despite goggle usage. Face shields that wrap around the face to the ears can be utilized in addition to goggles to provide increased protection from splashes and sprays for the entire face as opposed to simply the eyes. Requiring these forms of protection in the workplace can contribute to a reduction in daily work-related ocular injuries [
201,
202].
5. Dry Eye Disease and its Consequences
Dry eyes and external ocular surface disease are thoroughly addressed in the ophthalmology literature[1]. Different types of dry eye syndromes are described. There are many prescription and non prescription medications available for treating the symptoms of dry eyes[2] .
Yet, the external environmental factors causing dry eyes are only briefly discussed. After 9/11, many first responders and workers were exposed to toxic dust[3]. There was extensive research on cancer, which was the highest priority, but many of those exposed are suffering from the chronic symptoms of dryness, burning, redness, irritation and light sensitivity even decades later[4]. A parallel situation of severe health consequences overshadowing ophthalmic issues resulting from exposure to the burn pits in Iraq[5]. The soldiers and civilian workers may experience dry eyes, but research focuses on higher priority sequelae[6]. Even the recent Ohio train derailment demonstrates the danger of toxic chemicals to nearby residents. Lawsuits are being pursue, but ophthalmologists are not being called upon to describe the short or long-term consequences. Artificial tears are expensive and bring danger of infection[7]. It is hard to spend hours working at a computer and conducting everyday activities vital to functioning in the modern world when one’s eye are burning.
This paper begins to address these issues in an exhaustive scientific fashion.