Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
On the one hand, reactive oxygen species (ROS) are involved in the onset and progression of a wide array of diseases. On the other hand, these are a part of signaling pathways related to cell metabolism, growth and survival. While ROS are produced at various cellular sites, in cardiomyocytes the largest amount of ROS is generated by mitochondria. Apart from the electron transport chain and various other proteins, monoamine oxidases (MAO) has been proposed to modify mitochondrial ROS formation.
- ischemia
- reperfusion
- heart failure
- pulmonary hypertension
1. Monoamine Oxidase Isoforms
Two different isoforms of MAO are known, namely MAO-A and MAO-B, both of which are located at the outer mitochondrial membrane. Species-dependent cardiomyocytes express different MAO isoforms: in rats, MAO-A predominates in adulthood, [116,117] while in adult mice MAO-B dominates [118,119]. Interestingly, in rat hearts, MAO-B activity also predominates up to an age of 2–3 weeks [120], most likely since MAO-B expression increases under mechanical strain as compared to the quiescent situation [121]. Human cardiomyocytes contain both MAO isoforms, but with more, albeit moderate, expression for MAO-A [122,123]. In rat hearts, MAO activity is higher in the left compared to the right ventricle [124] and females have higher plasma MAO activity than males [125] as estrogens can modulate MAO activity [126].
2. MAO Substrates
The two MAO isoforms have common substrates such as dopamine, but also specific substrates: MAO-B can metabolize 1-methyl histamine [127], produced by the histamine-N-methyltransferase [128], while MAO-A metabolizes serotonin (or 5-hydroxytryptamin, 5-HT) and catecholamines (for review, see [129]). MAO requires flavin adenine dinucleotide as a cofactor that is reduced by the reaction of, and subsequently re-oxidized by, oxygen and water, generating hydrogen peroxide [130]. MAO can also form reactive aldehydes, such as 4-hydroxynonenal, as a byproduct of catecholamine metabolism through cardiolipin peroxidation inside mitochondria in primary cardiomyocytes. Deleterious effects of 4-hydroxynonenal are physiologically prevented by the activation of mitochondrial aldehyde dehydrogenase 2 [73].
Mice deficient in both MAO-A and MAO-B demonstrate increased tissue levels of serotonin, norepinephrine, dopamine, and phenylethylamine [131], and genetic ablation of MAO-A increases the serotonin concentration in the blood and tissue of rats [132]. Similarly, a blockade of MAO by drugs indicated for other uses (e.g., antidepressants) can alter histamine levels in mice hearts [133]. In contrast, MAO-A overexpression decreases the level of norepinephrine and serotonin in the heart (for review, see [134]) (Figure 2).
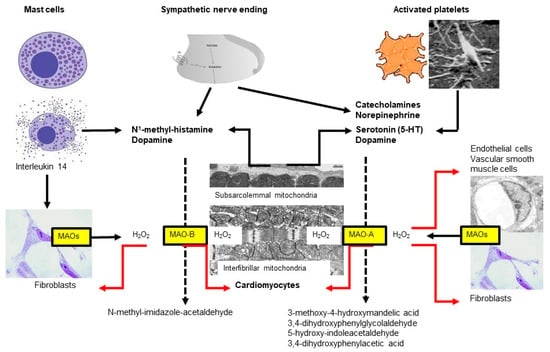
Figure 2. Two subtypes of monoamine oxidases (MAO)—named A and B—are located at the outer mitochondrial membrane, which differ in their substrate specificity. Almost all cell types express MAOs but the respective subtype might differ between species, organs and age. In the heart, MAOs are expressed in cardiomyocytes, fibroblasts, vascular smooth muscle and endothelial cells. In the heart, MAO substrates are derived from different sources including mast cells, sympathetic nerves, platelets and cardiomyocytes.
3. MAO Expression
An increased expression/activity of MAO occurs during aging [135,136] and with different diseases such as arterial hypertension [137,138], pulmonary hypertension [139,140], hypertrophy [141], diabetes [142,143,144,145], myocardial infarction [146] or heart failure [147,148]. In the streptozotocin-induced diabetic rat model, particularly the MAO-B isoform is induced in aortas and hearts and contributes to the generation of reactive oxygen species [142]. While the underlying mechanisms of MAO upregulation under the above conditions are still unclear, one potential factor contributing to increased MAO expression/activity in the heart might be increased substrate availability (for review, see [134]).
An increased sympathetic tone increases plasma norepinephrine and epinephrine concentrations, and increased norepinephrine spillover as seen in chronic heart failure patients [149,150]. Serotonin concentrations are increased during different disease states (for review, see [151]) and part of the increase has been attributed to altered platelet function [152]. Histamine co-localizes with norepinephrine in neurons [153] and is enclosed in cytoplasmatic granules of mast cells, which lie adjacent to blood vessels and between cardiomyocytes [154], and mast cell degranulation might occur under stress conditions [155]. Moreover, serotonin can be formed in the mouse and human heart [156], probably by cardiomyocytes themselves [157].
In AC16 cardiomyocytes, MAO-A mRNA and protein expressions are affected by non-coding RNAs since knockdown of the non-protein coding RNA 472 (LINC00472) reduced MAO-A expression, the results being partly abolished by miR-335-3p inhibition. Thus, LINC00472 positively regulates MAOA expression via interaction with miR-335-3p [158].
4. Monoamine Oxidases and Hypertrophy
Cardiac hypertrophy is a typical early adaptive response to increased cardiac workload and mechanical stress. However, in cases of prolonged or chronic stress, this response may become maladaptive and ultimately lead to heart failure. Cardiomyocytes synthesize additional sarcomeres, leading to the thickening of the ventricular wall and increased overall cardiac mass and size. The subcellular reorganization that underlies cardiomyocyte hypertrophy was found to require functional and responsive mitochondrial dynamics (for review, see [159]).
In wild type mice, pressure overload induced by transverse aortic constriction results in increased dopamine catabolism, left ventricular hypertrophy and dilation progressing to cardiac dysfunction. In contrast, in MAO-B knockout mice with transverse aortic constriction concentric left ventricular hypertrophy and function are maintained, both at the early (weeks) and late stages (months) [160]. As outlined above, in the hearts [137] as well as in the isolated cardiomyocytes [138] of spontaneously hypertensive rats MAO activity is significantly increased even before the development of cardiac hypertrophy [138]. Increased MAO activity might represent an early event in the development of cardiac hypertrophy [137] due to its potential impact on cardiac metabolism [161] since cardiac hypertrophy normally goes along with a metabolic switch to preferential use of carbohydrates rather than fatty acids [162,163].
In rat cardiomyocytes, administration of high micromolar concentrations of serotonin or dopamine increases glucose transport through the upregulation of glucose transporters 1 and 4 at the sarcolemma; the increase in glucose import is blocked by MAO-A inhibition [161]. At similar concentrations, serotonin induces cardiomyocyte hypertrophy, again the effect being largely attenuated by MAO-A inhibition [164] (or blockade of the extracellular regulated kinase). In addition, the effects of angiotensin II on hypertrophy are attenuated by a pharmacological blockade of MAO-A in rats [165]. Since lower concentrations of serotonin induce cardiomyocyte hypertrophy independent of MAO-A through the activation of the 5-HT(A2) receptor [166,167], genetic deletion of MAO-A also increases load-dependent ventricular hypertrophy [132] (for review, see [117]). Thus, both an increased or decreased MAO expression/activity can contribute to hypertrophic effects, depending on substrate availability.
5. Pulmonary Hypertension
MAOs have also been proposed to play an important role in pulmonary hypertension [168]. In rats, pulmonary hypertension secondary to monocrotaline injection [139], sugen5416/hypoxia, or pulmonary artery banding [140] upregulates MAO-A expression in the pulmonary vasculature and the failing right ventricle. Clorgyline treatment reduced the right ventricular afterload and pulmonary vascular remodeling in sugen/hypoxia rats through reduced pulmonary vascular proliferation and oxidative stress, resulting in improved right ventricular stiffness and relaxation and reversed right ventricular hypertrophy. In rats with pulmonary artery banding, clorgyline had no direct effect on the right ventricle [140]. In contrast, recent unpublished data demonstrate less myocardial structural or functional changes secondary to the pulmonary artery banding in cardiomyocyte-specific MAO B knockout mice in the right ventricle.
6. Monoamine Oxidases and Ischemia/Reperfusion (I/R) Injury
Under stress conditions such as I/R, the autonomic nervous system is activated, releasing neurotransmitters that are metabolized by MAOs, thereby directly influencing heart function [169]. Besides norepinephrine, serotonin and histamine also play important roles in I/R injury. Serotonin accumulates in the heart during ischemia [170] and is degraded after reperfusion depending on MAO-A activity after uptake into cells [171]. Mast cells become activated during stress conditions and release histamine [155]; histamine release from the heart is increased during I/R [127]. While mast cell activation thus increases substrate availability for MAO-B, MAO-B inhibition prevents mast cell degranulation in diabetic mice hearts [144], implying a vicious cycle of mast cell and MAO-B activation.
During 30 min ischemia, hydroxyl radical production increases 2-fold with a further increase upon 60 min reperfusion in isolated rat hearts, both of which can be decreased by pargyline administration. The decrease in ROS formation following MAO inhibition is associated with reduced cardiomyocyte injury following I/R [172]. Similarly, cardiomyocyte-specific MAO-B knockout reduces infarct size following I/R in isolated mice hearts [173].
In vivo, the inhibition of MAO-A largely reduces myocardial ultrastructural damage induced by 30 min ischemia and 60 min reperfusion in the rat heart, associated with the prevention of postischemic oxidative stress, neutrophil accumulation, and mitochondrial-dependent cell death [174]. Infarct size and cardiomyocyte apoptosis are also significantly decreased in MAO-A-deficient animals following 30 min ischemia and 180 min reperfusion, the protection being accompanied by sphingosine kinase 1 inhibition and less ceramide accumulation [175].
Cardioprotection can be achieved also by mechanical intervention such as ischemic preconditioning [176]. In both male and female rat hearts, ischemic preconditioning improves functional recovery following I/R, which is further enhanced in the presence of MAO inhibition by either clorgyline or pargyline. However, infarct size is similar among all preconditioned groups, regardless of the presence of MAO inhibitors, indicating that acute inhibition of MAOs potentiates the preconditioning-induced postischemic functional recovery without having any further effect on infarct size beyond that achieved by ischemic preconditioning [177].
7. Monoamine Oxidases and Left Ventricular Remodeling/Heart Failure
Rasagiline mesylate (N-propargyl-1 (R)-aminoindan) (RG), a selective, potent irreversible inhibitor of monoamine oxidase-B, administered for 28 days (2 mg/kg) starting 24 h after myocardial infarction, preserves left ventricular geometry and function. Treatment with rasagiline prevents tissue fibrosis and attenuates cardiomyocyte apoptosis in the border zone of the infarct associated with a markedly-decreased malondialdehyde level in the border zone, indicating a reduction in tissue oxidative stress [178]. Additionally, MAO-A is an important source of oxidative stress in the heart and MAO-A-derived reactive oxygen species contribute to dilated cardiomyopathy [145]. In mice, left ventricular function following four weeks of coronary artery occlusion improves by pharmacological or genetic inhibition of MAO-A. Both interventions protect the mice from 4-hydroxynonenal accumulation and mitochondrial calcium overload, thus mitigating ventricular dysfunction [73].
Furthermore, it has recently been suggested that upregulation of MAO-A during heart failure will accelerate intracellular catecholamine degradation, thereby inhibiting a direct stimulation of β-adrenergic receptors at the sarcoplasmic reticulum; this interaction is closely linked to phospholamban phosphorylation and calcium filling of the sarcoplasmic reticulum [179,180].
The importance of MAO for left ventricular remodeling and heart failure development can be shown in mice with chronic overexpression of MAO-A. Here, reactive oxygen species [136] and 4-hydroxynonenal concentrations increase, followed by mitochondrial dysfunction [181], cardiomyocyte hypertrophy, reduced left ventricular function and increased cardiac fibrosis [73], as well as increased cardiac inflammation [182] (for review, see [134,183]). When transgenic animals are treated with the antioxidant N-acetyl cysteine part of the above effects can be rescued [136,184].
Moreover, heart failure induced by doxorubicin is affected by MAO inhibition, which prevents both the severe oxidative stress induced by doxorubicin as well as chamber dilation and cardiac dysfunction in doxorubicin-treated mice in vivo [185].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24076459
This entry is offline, you can click here to edit this entry!
