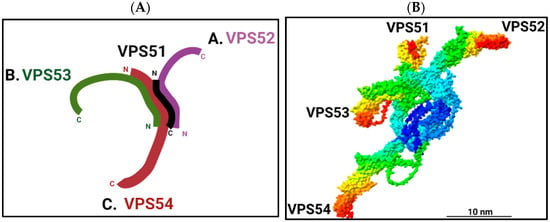
2. Functions of the GARP Complex
Although all GARP subunits are dispensable for life in yeasts [
1], deletion of GARP causes an increased protein secretion, abnormal autophagy, and defects in vacuolar morphology [
4,
5]. GARP deletion also causes infertility in worms [
54], a shorter life span in flies [
53], and embryonic lethality in mice [
55].
2.1. GARP as a Molecular Tether for the Endosomal-Derived Vesicles
One of the most studied functions of the GARP complex is its role in retrograde vesicular transport from endosomes to TGN, which enables the retrieval of recycling transmembrane proteins like vacuolar protein sorting receptor Vps10p in
S. cerevisiae. Vps10p binds to the pro-carboxypeptidase (Pro-CPY) in the TGN. Pro-CPY is one of the vacuolar hydrolase precursors that is delivered to the vacuole, while the receptor Vps10p needs to be recycled back to the TGN for further rounds of sorting. A mutation in any GARP subunit prevents Vps10p recycling to the TGN. Instead, Vps10p in GARP mutants is missorted to the vacuole and degraded. As a consequence of Vps10p degradation, GARP mutants missort precursors of vacuolar hydrolases, causing their secretion. Similarly, the recycling of TGN protein Kex2p and plasma membrane v-SNARE Snc1p is partially blocked in GARP mutants, resulting in their vacuolar degradation [
4].
In mammalian cells, cation-independent mannose 6-phosphate receptor (CI-MPR/IGF2R/MPRI) performs a function similar to Vps10p in yeast. Knockdown of mammalian GARP subunits prevented the retrieval of CI-MPR to the TGN, resulting in missorting and secretion of lysosomal hydrolases. Blockade in the sorting of lysosomal hydrolases developed enlarged lysosomes as a result of the accumulation of undegraded materials in the lumen of the organelle [
22].
There is also a blockage in the retrieval of TGN-localized recycling protein-TGN46 in GARP mutants [
28]. Moreover, the recycling Golgi resident proteins (GPP130, TMEM165, and TGN46) are significantly depleted in VPS54KO and VPS53KO cells [
56]. Similarly, the transport of the Shiga toxin is inhibited in the cells with siRNA-mediated KD of GARP subunits [
22].
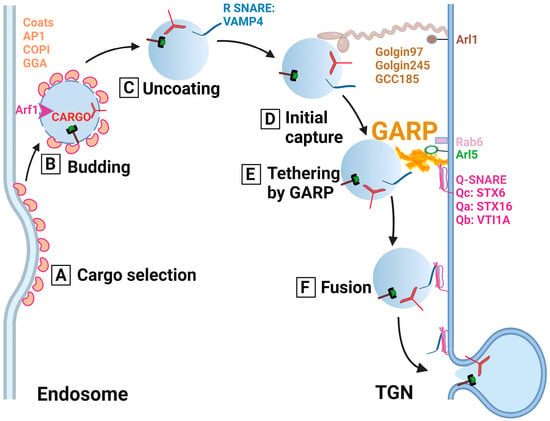
Based on GARP mutant phenotypes and on the similarity between GARP and other CATCHR complexes, it was proposed that GARP serves as a TGN-located molecular tether for the recycling of endosome-derived vesicles (
Figure 2). In support of this model, yeast vps54 mutant cells accumulate multiple vesicular structures [
2]. In addition, siRNA depletion of GARP subunits in HeLa cells caused redistribution of recycled CI-MPR to a vesicle-like “haze” [
22]. It will be essential to isolate and characterize GARP-dependent membrane carriers and investigate the interplay between GARP and other players of the TGN vesicle tethering/docking/fusion machinery.
Figure 2. Cartoon depicting the predicted role of the GARP complex and other trafficking components in the tethering of endosome-derived vesicles to the TGN. (A) Cargo selection: Recycling soluble and transmembrane cargo molecules such as the mannose-6-phosphate receptor and TGN46 are selected and packaged in the trafficking intermediates (vesicle) using ARF1 GTPase and a vesicular coat (AP1, GGA or COPI). (B) Budding: After the vesicle is formed, it is budded off the endosomal compartment. (C) Uncoating: ARF1 hydrolyzes GTP, and the coat gradually falls off. (D) Initial capture: Once the vesicle reaches proximity to the TGN, coiled-coil tethers (Golgin97, Golgin245, and GCC185) perform the initial capture. (E) Tethering by GARP: GARP complex binds coiled-coil tethers and SNAREs to control and coordinate docking of the vesicle with the TGN membrane. (F) Fusion: Following the tethering by the GARP complex, the trans-SNARE complex is formed, which results in vesicle fusion with the TGN to deliver the recycling molecules.
2.2. GARP as a Regulator of SNARE Complexes
Like other MTC vesicular tethers [
30,
57,
58,
59,
60] the GARP complex physically interacts with a specific subset of SNARE molecules. Mammalian GARP complex interacts with endosome-TGN STX16 SNARE complex (STX16/STX6/VTI1A/VAMP4), and GARP KD cause decreased formation and/or stability of this complex [
28]. In addition, GARP has been shown to regulate the localization and stability of intra-Golgi Qb-SNARE GOSR1 and Qc SNARE BET1L. These v-SNAREs work in the STX5/GOSR1/BET1L/YKT6 SNARE complex to facilitate the fusion of intra-Golgi recycling vesicles. Immunofluorescence microscopy demonstrated the depletion of Golgi GOSR1 and BET1L signals in GARP-KOs. Consistently, the total protein abundance of v-SNAREs in the GARP-KOs was significantly decreased [
61]. This indicates that the GARP is involved in regulating of at least two different SNARE complexes promoting the fusion of cargo vesicles not only in TGN but also in the early-Golgi compartments.
2.3. Role of the GARP Complex in the Maintenance of Golgi Glycosylation Machinery
Glycosylation of proteins and lipids contributes to a variety of biological processes, and abnormal glycosylation in humans is a hallmark of many diseases. Mutations in proteins that are directly or indirectly involved in the glycosylation process can cause congenital disorders of glycosylation [
62,
63]. The glycosylation machinery is localized in the ER and Golgi [
64], and therefore it was initially assumed that post-Golgi trafficking does not significantly contribute to the glycosylation process. Mutations in the GARP complex and GARP-associated clinical cases of glycosylation disorders have not been widely known. However, a recent publication described a 6-year-old patient with a neurodevelopmental disorder having a mutation in the VPS51 subunit of GARP/EARP complex and an abnormal pattern of glycosylation [
65].
Similarly, the study in our lab revealed that KOs of GARP complex subunits VPS53 and VPS54 in different types of tissue culture cells (hTERT–RPE1, HEK293T and HeLa) [
66] resulted in severe defects in both
N- and
O-protein glycosylation. Golgi glycosylation enzymes (MGAT1, B4GALT1, and ST6GAL1) were also significantly depleted. Retention using selective hooks (RUSH) assay showed that B4GALT1 was not retained at the Golgi complex in GARP-KO cells but was missorted to the endolysosomal compartment. Another
trans-Golgi enzyme, ST6GAL1, was also not retained in the Golgi in GARP-impaired cells. It was shown that the defect in Golgi enzymes retention/recycling depends entirely on GARP and not on the EARP complex. The malfunction, mislocalization, and reduced stabilization of Golgi enzymes associated with a glycosylation defect were found to be rescued upon expression of the missing GARP subunit. Taken together, we concluded that GARP plays a crucial role in normal Golgi glycosylation by mediating the maintenance of the Golgi glycosylation machinery [
56].
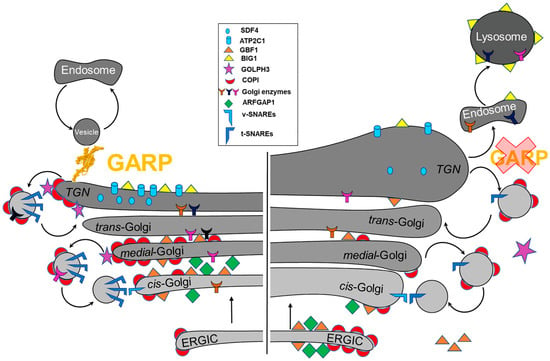
2.4. Role of the GARP Complex in Normal Golgi Physiology
Although the majority of GARP-sensitive proteins are predicted to reside in
trans-Golgi compartments [
56], the proteomic analysis of Golgi-enriched membranes in hTERT-RPE1 cells lacking GARP subunits revealed significant depletion of
cis/
medial Golgi proteins such as GLG1/MG-160, GALNT1, and MAN1A2 [
61]. Recent results from our lab revealed that in human GARP KO cells, COPI vesicular coat complex, which is involved in intra-Golgi and Golgi-ER retrograde trafficking [
67], is partially mislocalized to the ERGIC compartment. In GARP KO cells, the COPI accessory proteins GOLPH3, ARFGAP1, and GBF1 were displaced from the membrane, while BIG1 was relocated to the endolysosomal compartment. In addition, transmission electron microscopy revealed dysmorphic Golgi features in GARP mutants. In VPS54 KO cells, there is an enlargement of the Golgi structure, indicating an increase in the distance between the cisternae. In VPS53 KO cells, the Golgi structure was round, swollen, and disrupted [
61]. This suggests that GARP dysfunction impacts not just
trans-Golgi compartments but also the entire Golgi complex (
Figure 3). Therefore, the GARP activity is required for normal Golgi physiology.
Figure 3. GARP dysfunction results in the depletion and mislocalization of multiple Golgi proteins, altering Golgi homeostasis. (Left) A schematic of Golgi proteins in wild type cells. (Right) GARP deficient cells are depleted for multiple resident Golgi proteins. COPI coats and ARFGAP1 are mislocalized to ERGIC., while BIG1 is relocated to endolysosomal compartments.
2.5. GARP and Lipid Homeostasis
Fröhlich et al. identified a crucial role for the yeast GARP complex in lipid homeostasis. Deletion of any component of the GARP complex resulted in the buildup of sphingolipid intermediates and long-chain bases, whereas pharmacological inhibition of sphingolipids can repair all observed GARP-KO mutant abnormalities. This shows that sphingolipid accumulation is the problem-causing factor in GARP-deficient cells [
68]. Maintenance of the phospholipid’s asymmetric distribution from the outer to the inner leaflet of the plasma membrane (PM) is done by phospholipid transporter flippases. The mislocalization of PM localized flippases results in changes in lipid homeostasis [
69]. Mutation in the GARP in yeast showed hypersensitivity to the phosphatidylethanolamine (PE) binding lantibiotic Ro by preventing cell growth. GARP mutants showed the localization of Dnf1p and Dnf2p to the vacuole, while in WT, it is localized to the PM. Vacuolar proteomics and lipidomics demonstrate missorting of flippases Dnf1p and Dnf2p, suggesting that remodeling of the lipid composition is one of the first occurring defects in GARP-depleted cells [
70]. A study in flies showed that the GARP complex is involved in sterol transport, and knockout of Vps54 resulted in reduced lifespan and impaired arborization of
Drosophila melanogaster. Significantly, the expression of a wild type copy of Vps54 in Vps54 KO flies rescued these mutant phenotypes [
71].
The GARP complex is also involved as a regulator in the morphogenesis of
Candida albicans. A peculiar feature of
Candida albicans is the switch between yeast and filamentous hyphal form in response to cues. A genomic screen in
Candida albicans revealed that deletion of GARP subunits impaired filamentation in response to Hsp90 inhibition. This filamentation defect was found to be due to the disruption of lipid homeostasis in the GARP mutant. GARP mutants have an increase in the number and size of lipid droplets compared to the control [
72].
Dysfunction of the GARP complex resulted in missorting of lysosome resident protein NPC2, responsible for the egress of LDL-derived cholesterol from lysosomes. As a result, GARP-depleted cells have an accumulation of cholesterol. This phenotype is similar to Niemann–Pick type C disease, which is a neurodegenerative disease characterized by a massive accumulation of free cholesterol and other lipids in almost all cells throughout the body, particularly in the brain and liver [
73].
2.6. Role of GARP Complex in the Secretory Pathway
Besides the endosomal-TGN trafficking function of the GARP complex and the promotion of SNARE function in membrane fusion, VPS52 has also been implicated in the regulation of the secretory pathway. The secretion of soluble secretory protein ss-sfGFP (super-folder GFP fused to signal sequence) was significantly decreased in VPS52 KO MDCK cells with subsequent accumulation of ss-sfGFP in lysosomes. This phenotype was prevented by the stable expression of VPS52. The functional importance of the
C-terminal region of VPS52 for restoring secretory phenotypes, as well as for the sorting of lysosomal proteins, was assessed where amino acids 411 to 556 are required for ss-sfGFP sorting and secretion while amino acids 411 to 723 are required for efficient lysosomal protein sorting [
74].
KO of GARP subunits in HEK293T cells caused severely defective anterograde transport of both glycosylphosphatidylinositol (GPI)-anchored and transmembrane proteins from the TGN [
75]. Overexpression of VAMP4, TMEM87A or its close homolog TMEM87B in VPS54 KO cells partially restored endosome-to-TGN retrograde transport and anterograde transport of GPI-anchored proteins. The authors proposed that GARP- and VAMP4-dependent endosome-to-TGN retrograde transport is required for the recycling of molecules critical for efficient post-Golgi anterograde transport of cell-surface integral membrane proteins.
2.7. Hijacking of GARP by Intracellular Pathogens
To determine the host components essential for monkeypox virus infection, genome-wide insertional mutagenesis was done in HAP1 human cells. The screening identified the significance of the GARP complex in the generation of extracellular viruses. Extracellular viruses are responsible for cell-to-cell and long-distance virus transmission, both of which are needed for pathogenicity. In GARP KO (VPS52 KO and VPS54 KO) cells, the virus yield was drastically reduced, whereas the structure of the mature virion was unaffected. Rescuing the knockout with the expression of the WT copy of the affected GARP subunit restored the virus yield. Interestingly, the electron microscopy revealed a decrease in the membrane wrapping in GARP KO cells, indicating that GARP could be used for efficient membrane wrapping of the mature virion. In addition, confocal microscopy demonstrated that the GARP KO infected cell lacks actin tail production compared to controls which is one of the mechanisms for viruses’ escape. The role of Golgi trafficking machinery in the production of monkeypox and vaccinia virus is further supported by another study in COG KO cells where COG KO cells produced lower virus yield and possessed a reduced number of actin tails compared to WT [
76,
77].
2.8. Future perspectives in GARP studies
Despite being discovered over two decades ago, our understanding of the GARP complex remains limited. To fully comprehend its function, it is crucial to investigate the complex's dynamic nature and elucidate its overall and subunit architecture, especially for the mammalian GARP complex in both free and membrane-bound states. Understanding how GARP switches from a "close" to an "open" conformation to tether incoming transport intermediates is critical.
The shared VPS51/52/53 trimer between the GARP and EARP complexes raises questions about how differences in one subunit between these complexes affect their localization and function. It is unclear whether the shared trimer is dynamically switching between GARP and EARP conformations or if the two complexes are stable and do not exchange subunits. Additionally, identifying the factors that make GARP unique from EARP, such as SNAREs, coiled-coil tethers, and small GTPases, is necessary.
Although defects in GARP subunits disrupt endosome-TGN trafficking, studies have shown that mutant phenotypes extend beyond this, affecting glycosylation, intra-Golgi transport, and secretion. Understanding the immediate and secondary impacts of GARP dysfunction is crucial.
Direct evidence of GARP's ability to capture specific transport vesicles is still lacking. It is unclear whether GARP captures vesicles independently or in conjunction with coiled-coil tethers and whether it captures only one type of transport vesicle. Identifying the specific protein and lipid cargo that relies on the GARP complex is critical. Isolating and further characterizing GARP-dependent membrane carriers and exploring the interplay between GARP and other players in TGN vesicle tethering, docking, and fusion machinery is essential.
While it is suggested that GARP promotes the assembly of TGN SNAREs, the exact mode of interaction between GARP and SNAREs is unclear. Further research is needed to elucidate the precise mechanisms by which GARP functions in the cell, such as whether it only interacts with the STX16 SNARE complex or binds and regulates the STX16-interacting SM protein VPS45.
The dysfunction of the GARP complex can result in pathogenesis in humans, mice, and plants. VPS54 null causes embryonic lethality in mice, raising questions about the specific functions of GARP in different cell types and how its dysfunction can lead to such severe outcomes. Furthermore, it is unclear why GARP deficiency primarily affects neuronal function and how the complex is involved in various signaling pathways associated with cancer. To gain a more comprehensive understanding of GARP functions and dysfunctions, it is imperative to study the complex in a range of human tissues and other species beyond yeast and mammalian cell models.