Impaired cognition is the primary symptom of dementia, which can lead to functional disability and reduced quality of life among an increasingly ageing population. Ageing is associated with increased oxidative stress, chronic low-grade systemic inflammation, and endothelial dysfunction, which reduces cerebrovascular function leading to cognitive decline. Chronic low-grade systemic inflammatory conditions, such as obesity, exacerbate this decline beyond normal ageing and predispose individuals to neurodegenerative diseases, such as dementia. Capsaicin, the major pungent molecule of chilli, has recently demonstrated improvements in cognition in animal models via activation of the transient receptor potential vanilloid channel 1 (TRPV1). Capsaicin-induced TRPV1 activation reduces adiposity, chronic low-grade systemic inflammation, and oxidative stress, as well as improves endothelial function, all of which are associated with cerebrovascular function and cognition.
1. Capsaicin
The capsaicinoids are the primary pungent molecule found in plants belonging to the
Capsicum genus, particularly chillies [
86]. They are a group of phenolic compounds characterised by a common vanilloid ring (
Figure 1) [
87]. Capsaicin, dihydrocapsaicin, nordihydrocapsaicin, homocapsaicin and homodihydrocapsaicin all comprise the capsaicinoids, with capsaicin being the most abundantly occurring of these [
87,
88]. Capsaicin has been extensively studied for its multiple benefits as an anti-carcinogenic, anti-inflammatory, antioxidant, and anti-obesity agent and for its use as a topical analgesic [
89,
90].
Table 1 summarises research studies that have investigated these effects.
When taken orally, capsaicin is passively absorbed in the stomach and jejunum [
26,
87]. Albumin transports capsaicin in the blood, and its vanilloid ring has a high affinity for the transient receptor vanilloid 1 (TRPV1), a non-selective cation channel [
87,
91]. TRPV1 receptors are widely expressed in the body and are found to be concentrated in neural tissue (peripheral and central) and the endothelium [
83]. When capsaicin binds to TRPV1, it causes a cation influx, activating numerous physiological pathways which are important modulators of vasodilation and inflammation [
92,
93,
94]. In turn, downregulation of these pro-inflammatory pathways and upregulation of anti-inflammatory pathways promote the increased expression of eNOS and, therefore, increased NO production and availability, counteracting the effects of endothelial dysfunction [
93,
95,
96].
Capsaicin: A Brief Overview of Its Role as Anti-Cardiometabolic Disease Treatment
Capsaicin’s action in reducing obesity, oxidative stress, and inflammation and improving cardiovascular function in animals has been previously described [
83,
86,
105]. Obesity increases blood triglycerides, free fatty acids and low-density lipoprotein (LDL), contributing to eNOS dysregulation, vascular remodelling, and atherosclerosis, which is the leading cause of cardiovascular and cerebrovascular disease [
83,
93,
106].
Capsaicin increases thermogenesis, energy expenditure and fat oxidation, all of which assist in decreasing adiposity [
83,
107]. This is achieved by the activation of TRPV1 and subsequent reduction of pro-inflammatory cytokines, such as TNF-α and IL-6, which are increased with greater adiposity [
27,
78,
108]. Ma et al. [
109] found that dietary capsaicin (0.1%) for 24 weeks in C57BL/6J mice activated TRPV1, inducing cytosolic calcium and reduced lipid accumulation and atherosclerosis [
106]. Wang, Y. et al. [
110] cultured human umbilical cord endothelial cells and treated them with capsaicin. Capsaicin increased NO, and reduced cytokine production, monocyte adhesion, adhesion molecule expression and activated NF-ĸB, thereby reducing inflammation. TRPV1 activation with 1 µM capsaicin rescued impaired macrophage autophagy induced by oxidised low-density lipoprotein, activating AMPK signalling, inhibiting foam cell formation, and preventing atherosclerotic plaque formation [
106]. Dai et al. [
111] fed male apolipoprotein E knock-out mice 0.01% capsaicin for 18 weeks alongside a high-fat diet or a high-fat diet with broad-spectrum antibiotics. Capsaicin reduced serum lipopolysaccharide (an inflammatory mediator) and low-density lipoprotein, as well as increased high-density lipoprotein. This was not observed in the group fed capsaicin with concurrent antibiotics. The improvements resulted in reduced intestinal inflammation and permeability, as well as improved endothelial function, which led to a significant reduction in atherosclerotic lesions. This demonstrates capsaicin’s ability to reduce adiposity and its associated inflammatory mediators, as well as reducing cardiovascular-induced risk factors that can reduce cerebrovascular function and cognition. This may also indicate capsaicin’s application as a resolution to other inflammatory diseases such as autoimmune diseases, gastrointestinal disorders, and other haemodynamic conditions, such as atherosclerosis.
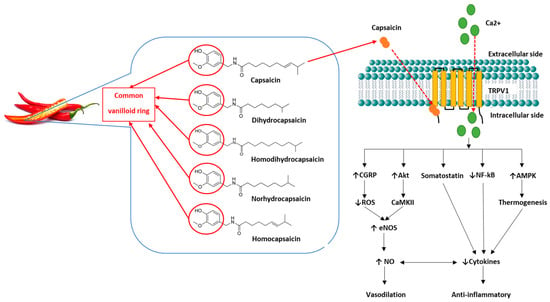
Figure 1. Capsaicin structure and function. Capsaicin and dihydrocapsaicin contribute the most to the pungency of chilli and are found primarily in the fruit pod [
86]. The capsaicinoids are characterised by their common vanilloid ring (circled). Capsaicin consists of a
trans configuration with a double-bond and an even number of branched-chain fatty acid moieties [
89]. The vanilloid ring binds intracellularly to TRPV1 channels on cell membranes, causing an influx of extracellular calcium into the cell and triggering numerous physiological pathways [
82,
94]. Capsaicin causes the release of sensory neuropeptides such as calcitonin gene-related peptide (CGRP) [
112]. CGRP is a potent vasodilator that reduces reactive oxygen species (ROS) production by promoting increased endothelial nitric oxide synthase (eNOS) function and nitric oxide (NO) production [
94,
112]. Somatostatin reduces pro-inflammatory cytokines, inducing anti-inflammatory and immunomodulatory effects [
112]. Phosphorylation of serine/threonine kinase 1 (Akt), mediates calcium-dependant protein kinase II (CaKMII), and increases phosphorylation of eNOS, thereby increasing NO production and vasodilation [
93,
113]. Adenosine monophosphate-activated protein kinase (AMPK) increases muscular uptake of glucose, thereby reducing adipose cytokine release, inducing anti-inflammatory effects [
83,
114]. TRPV1 also regulates transcription of nuclear factor kappa B (NF-ĸB), therefore assisting modulation of cytokine transcription factors and reducing inflammation [
94]. Abbreviations: ↑: increased; ↓: decreased.
2. The Effects of Capsaicin on Cognition and Cerebrovascular Function
2.1. Cognition in Animal Studies
Capsaicin’s action in reducing obesity, oxidative stress, and inflammation and im-proving cardiovascular function in animals has been previously described [
83,
86,
105]. Obesity increases blood triglycerides, free fatty acids and low-density lipoprotein (LDL), contributing to eNOS dysregulation, vascular remodelling, and atherosclerosis, which is the leading cause of cardiovascular and cerebrovascular disease [
83,
93,
106].
Tau proteins are concentrated in the central nervous system and are involved in microtubule assembly [
115]. Abnormal tau proteins can increase phosphorylation and decrease microtubule binding, forming amyloid oligomers (such as amyloid-beta, Aβ) or aggregated deposits, which impair brain function by reducing intra- and inter-neuronal signalling, leading to cognitive decline [
116]. Intraperitoneal administration of 1 mg/kg of capsaicin for two weeks restored Aβ-induced memory deficits via improved hippocampal synaptic function in C57BL/6 mice. This possibly occurred because it increased the expression of the neuroprotective protein postsynaptic density protein 95 (PSD95), which is often reduced with AD [
117]. The increase in PSD95 improved spatial learning in adult C57B1/6 mice, as its primary role is to maintain synaptic plasticity and promote inter-neuronal signalling. Balleza-Tapia et al. [
24] also found that hippocampal homogenate with tissue-bath perfusion of capsaicin significantly reduced levels of Aβ and tau protein via activation of TRPV1 in mice. Intraperitoneal administration of 1 mg/kg of capsaicin in an AD mice model upregulated TRPV1, alleviated AD-type pathologies and improved spatial learning and memory [
118]. Shiri et al. [
23] found that a 10 mg/kg single dose of capsaicin given intraperitoneally improved cognitive performance via TRPV1 in rats, as measured by passive avoidance learning tests. Pegorini et al. [
22] found a single dose of capsaicin between 0.2 and 0.6 mg/kg capsaicin, administered via sub-cutaneous injection, was neuroprotective. The authors reported that capsaicin increased the survival rate of CA1 neurons in the hippocampus seven days post-injection in male Mongolian gerbils. However, low-dose capsaicin (0.1 mg/kg) did not affect cognition. Abdel-Salam et al. [
25] reported that either 25 mg or 50 mg/kg/day capsicum extract (1.2% capsaicin) given for 30 days improved memory performance and increased central NO concentration, as well as reduced markers of oxidative stress, inflammation and neurodegeneration in a rat model of AD. They also reported that 50 mg/kg/day of capsicum extract reduced oxidative stress and inflammation in non-AD rats compared to the control group.
Cholinesterase enzymatically breaks down acetylcholine, which is a powerful cholinergic vasodilatory neurotransmitter that declines with ageing [
119,
120]. Cholinesterase is increased in AD, further reducing acetylcholine. Therefore, cholinesterase inhibitors are a current first-line treatment of AD pathologies [
121,
122]. Rajashri et al. [
123] found 13 days of dietary chilli oleoresin containing capsaicin (50 mg/kg; 1.9%
w/
w) given with scopolamine (an anticholinergic), reduced acetylcholinesterase (AChE) by 50%. Scopolamine alone decreased AchE, however, in the absence of a capsaicin only arm, it is unclear whether this was a result of capsaicin promoting scopolamine’s actions. Viayna et al. [
124] also reported that capsaicin (2 mg/kg intraperitoneally, three times per week for four weeks), scaffolded with the cholinesterase inhibitor huprine Y significantly reduced the Aβ42/Aβ40 ratio in the hippocampus. The reduction in this ratio improved spatial learning and memory and decreased neuroinflammation and hippocampal oxidative stress in APP/PS1 mice. Shalaby et al. [
125] reported that 47 days of intragastric infusion of capsaicin at a dose of 10 mg/kg in mice significantly ameliorated Aβ1-42 peptide and tau proteins in the hippocampus, abolishing behavioural impairments. A capsaicin-rich diet (0.01%, approximately 30 mg/kg capsaicin) for six months improved spatial learning and memory consolidation in an AD mice model [
27]. This showed that chronic capsaicin intake reduced the total Aβ burden by 32.3% and significantly attenuated tau hyperphosphorylation in both the neocortex and hippocampus. Wang, J. et al. [
27] also found that capsaicin significantly reduced proinflammatory cytokines IL-6 and TNF-α, and improved the expression of neuroprotective post-synaptic proteins, such as PSD95, thereby ameliorating neuroinflammation.
cAMP-response-element-binding-protein (CREB), a transcription factor critical in maintaining spatial and long-term memory, is downregulated in AD [
126]. Increased phosphorylation of CREB is linked to the binding of calcitonin gene-related peptide (CGRP), a potent vasodilator [
127,
128]. Intragastric administration of a single dose of capsaicin (10 mg/kg) increased the expression of CREB [
129]. Furthermore, 1 m/kg subcutaneous administration of capsaicin for eight days increased CGRP tissue levels in the hippocampus [
130]. Therefore, capsaicin increased spatial memory and cognitive performance [
129,
130]. This action was supported by Bashiri et al. [
131], who found that intrahippocampal capsaicin injections (0.05; 0.1; or 0.3 µg/rat) augmented mRNA expression of cyclic adenosine monophosphate (cAMP) and TRPV1 in the CA1 area of the hippocampus, improving memory in rats with biliary cirrhosis.
Avraham et al. [
132] found improvements in neurological scores and cognition up to 14 days following a single dose intraperitoneal injection of capsaicin (1.25 µg/kg) in female Sabra mice with hepatic failure. Further, these effects were reversed with the application of a TRPV1 antagonist, confirming that the observed effects of capsaicin were vanilloid mediated [
132].
Together these results demonstrate capsaicin’s ability to reduce oxidative stress and inflammation centrally and systemically via TRPV1, thereby alleviating cognitive deficits in animal models of disease. As these findings are all associated with reduced inflammation, it is logical to conclude that the underlying mechanisms of ED could also influence cognitive performance, and these findings could therefore translate to the human cerebrovasculature.
2.2. Cognition in Human Studies
Only one study assessed the effects of capsaicin on cognition. Liu et al. [
133] assessed the chilli pepper consumption of 338 community-dwelling people (>40 years old) from Chongqing, China, using a self-reported food frequency questionnaire. Cognition was measured using a Chinese version of the Mini-Mental State Examination (MMSE). A capsaicin-rich diet was positively correlated with significantly higher MMSE scores. However, those who consumed chilli daily were younger than those who self-reported weekly chilli consumption. This was attributed to the social phenomenon that older people preferred bland diets. Although limitations of this study also include the inability to quantify self-reported chilli consumption, this demonstrates the possibility that capsaicin could influence human brain health, and that further studies are required to test the effects of chronic chilli consumption on cognition.
2.3. Cerebrovascular Function in Animals and Humans
Limited studies have investigated the effect of capsaicin on cerebrovascular function. In vitro studies of feline MCAs found vasodilation of pial arteries occurred with a lower dose of capsaicin (5 × 10
−8 M) compared to high dose capsaicin (3 × 10
−7 M) which had vasoconstrictive outcomes [
134]. More recently, Marics et al. [
135] tested the dural application of capsaicin on meningeal blood flow in vivo, using a laser Doppler flowmeter positioned over a branch of the middle meningeal artery of rats. CGRP release from the meningeal afferents was elevated in response to both low (10 µM) and high (100 µM) topical capsaicin application on the dura mater. Further, CGRP release was seen to be higher in obese rats than in control rats [
135]. This may be due to decreased CGRP receptor sensitivity to CGRP (i.e., increased resistance of the CGRP receptor to CGRP) [
136]. Xu et al. [
137] reported that six months of dietary capsaicin (0.01% for mice and 0.02% for rats) increased the phosphorylation of eNOS in carotid arteries which was associated with the activation of TRPV1. Marquez-Romero et al. [
138] pipetted escalating capsaicin dosages (33, 66, 99, 132, 165 µM) onto filtered paper. A single application was applied for 20 min to the hemi-palate of 30 healthy undergraduate students and CBF was measured using TCD. Capsaicin increased CBF, thus demonstrating its potential to elicit vasodilatation. Given the link between CBF and cognition and the limited studies performed using capsaicin, more studies are needed to determine the effects of capsaicin on cerebrovascular function.
Recently, the synthetic capsaicin analogue, nitro-capsaicin, has been studied for its effect on the brain structure and cognition [
139]. Nitro-capsaicin substitutes the OCH
3 group on capsaicin with NO
2 and produces less gastro-intestinal irritation compared to capsaicin [
139,
140]. Jamornwan et al. [
140] found that nitro-capsaicin, in both vascular damaged and control microglial cell cultures, suppressed microglial activation, decreasing proinflammatory cytokines, such as TNF-α and IL-6, and enhanced anti-inflammatory factors, such as IL-4 and IL-10. Further, when mice with aberrant e4 apolipoprotein E (ApoE4) genes were given 1 mg/kg/day intraperitoneal capsaicin for 1 month, it reversed impaired lipid metabolism, microglial dysfunction, and other neuronal impairments induced by mutant ApoE4 [
141]. This demonstrates the potential of capsaicin and capsaicin analogues to reduce chronic microglial activation, a risk factor of neurodegenerative disease and cognitive decline.
This entry is adapted from the peer-reviewed paper 10.3390/nu15061537