Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Neurodegeneration is hallmarked by the progressive loss of dopaminergic neurons and/or a significant increase in protein aggregates in the brain. Neurodegenerative diseases are a leading cause of death worldwide with over 15 million people currently suffering from either Parkinson’s disease (PD) or Alzheimer’s disease (AD). PD is often characterized by both motor and non-motor symptoms, including muscle rigidity, tremors and bradykinesia, with AD displaying symptoms of confusion and dementia.
- Parkinson’s
- Alzheimer’s
- neurodegeneration
1. Introduction
The hallmark of neurodegeneration is the progressive loss of brain function, often with overlapping biological and clinical symptoms [1]. In general, neurodegenerative diseases share common pathologies and pathways, most prominently protein aggregation, oxidative stress, neuroinflammation and blood–brain barrier dysfunction, leading primarily to the death of neurons from various regions of the brain [2,3,4]. Neuronal degeneration is profound in both Alzheimer’s (AD) and Parkinson’s disease (PD) with numerous overlapping pathways implicated, such as regional aggregation of intracellular proteins Tau and Alpha-synuclein [5]; complex genotype–phenotype relationships with common mutations such as leucine-rich repeat kinase 2 (LRRK2), PTEN-induced kinase 1 (PINK1) and amyloid precursor protein (APP) [6]; and alterations in pathways such as autophagy–lysosome activity, creating an imbalance between autophagosome formation and the autophagic degradation usually involved in clearing aggregated proteins [7]. Mitochondrial homeostasis is also implicated in AD and PD, leading to neuronal cell death via an increase in oxidative stress [8,9]. Lastly, innate immunity, synaptic toxicity and network dysfunction all contribute to the neuronal loss observed in AD and PD [10] (Figure 1).
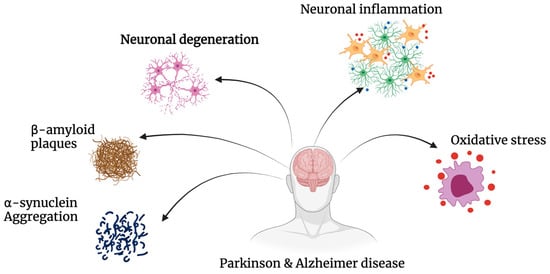
Figure 1. Pathogenesis of Alzheimer’s’ disease and Parkinson’s’ disease. AD and PD share common pathways of degeneration. Both neurodegenerative diseases exhibit significant amounts of oxidative stress, neuronal inflammation and degeneration, as well as the build-up of insoluble proteins including β-amyloid and α-synuclein.
AD and PD are the two most common neurodegenerative diseases worldwide. Cognitive dysfunction is the primary symptom exhibited in AD, while significant motor dysfunction is cardinal to PD [5]. AD is the most common cause of dementia, with a prevalence estimated at 24 million worldwide, which is expected to continue to rise over the next decade (GBD 2019 Dementia Forecasting Collaborators 2022). AD affects around 11% of the population over the age of 65, with PD affecting 2–3% of the population over 65 years of age, becoming the second most common neurodegenerative disease after AD [11]. AD can be classified into four stages: pre-clinical/pre-symptomatic, mild/early stage, moderate stage and severe/late stage [12,13,14]. These stages are often characterised by progressive memory loss, impaired balance, aphasia-like symptoms and an overall lack of independence in carrying out activities of daily living [15,16,17]. Two neuropathological changes have been identified in AD; positive lesions and negative lesions. Accumulation of neurofibrillary tangles, amyloid plaques and other deposits are significant indicators of AD, while on the other hand, neuronal, neuropil and synaptic loss-induced atrophy is also indicated in AD [18,19]. While the precise cause of the underlying pathological changes in AD is unknown, risk factors including age, genetics, traumatic brain injuries, diet and immune system dysregulation are some of the key contributors [20].
PD is associated with a lack of dopamine and an overall slowing of movement (bradykinesia) along with either a resting tremor or rigidity [21]. It has been suggested that there are two stages to PD, early (1 and 2) and late (3 and 4). In the early stages, symptoms can include rapid eye movement sleep behaviour disease (including sleep paralysis), as well as decreased smell, suggesting onset in the medulla and olfactory bulb. In stages 3 and 4, symptoms are more typical of cognitive impairment, including issues with movement and gait as well as hallucinations, suggesting that this stage’s pathology has progressed to the substantia nigra pars compacta and other midbrain and basal forebrain regions [22,23], often with aggregates of alpha-synuclein [23].
2. Aetiology and Pathophysiology of Disease
PD and AD are characterised by the presence of insoluble protein deposits, β-amyloid plaques and tau-containing neurofibrillary lesions in AD and α-synuclein-rich Lewy bodies in PD (Figure 1). Neuropathological changes in the disease progression and pathology of AD include neurofibrillary tangles, amyloid plaques, dystrophic neurites and neuropil threads [24]. PD presents with abnormal α-synuclein aggregates and the presence of Lewy bodies with selective loss of dopaminergic neurons [25,26]. Typically, PD is diagnosed at the mid to late stage of the disease due to a long period of dormancy between the initial loss of dopaminergic neurons and the development of more “typical” clinical symptoms [21,27]. AD follows a similar pathway with initial symptoms presenting over a 2–4-year period, then progressively worsening over the subsequent 10 years. PD and AD are largely considered to be sporadic, with evidence in recent years continuing to support the hypothesis that AD and PD may have substantial genetic components. In PD, this includes reported defects in the SNCA, PINK1 genes (associated with abnormal mitochondria and increased apoptosis), parkin (associated with impaired damaged protein tagging with ubiquitin), LRRK2 (associated with increased neuroinflammation) and DJ-1 (associated with increased reactive oxygen species), with genes such as APP (associated with the generation of beta-amyloid peptides), PSEN1 and PSEN2 (both interact with APP and are associated with the overproduction of toxic beta-amyloid peptides) displaying defects in AD [28,29,30,31].
Taking a closer look at both AD and PD suggests many commonalities in their pathophysiology, with many genes shared and co-expressed [32], as well as stark differences [33]. AD is well known for its increased concentration of Aβ42 (β-amyloid 42—due to mutations in the APP gene) which encourages the production of oligomers (that are neurotoxic). These oligomers cluster and eventually form plaques that contribute to symptoms presented above for AD [34,35]. Tau, usually functioning to stabilise axonal microtubules, is abnormally phosphorylated in AD. When this occurs, these highly phosphorylated tau proteins tend to clump together into filaments and further into insoluble neurofibrillary aggregates, spreading throughout the brain [36,37]. Tauopathy is also closely linked to granulo-vacuolar degeneration (GVD) in AD patients. Granulo-vacuolar bodies (GVBs) are often present in hippocampal pyramidal cells of those with AD, which is likely associated with cognitive decline in patients [34,38,39].
The increased loss of neurons in the substania nigra pars compacta (mostly), combined with the presence of abnormal aggregates, underpins the pathophysiology of PD [40]. Losing these neurons, predominately dopaminergic neurons, reduces dopamine levels, thus leading to the symptoms [41]. Drilling down into mechanisms, alterations in α-synuclein lead to either an increase in aggregation of protein or diminished capacity for its degradation, creating fibrilization of α-synuclein in Lewy bodies or neurites promoting neurodegeneration [42,43]. Furthermore, this α-synuclein accumulation directly increases levels of mitochondrial stress and reactive oxygen species [44,45]. Parkin is associated with many subcellular compartments such as synaptic vesicles and the endoplasmic reticulum, as well as playing a neuroprotective role via mitochondria by delaying mitochondrial swelling and the activation of caspase 3 [46]. Its function is closely linked to the ubiquitin-proteasome proteolytic pathway. Parkin is an E3 ubiquitin-protein ligase that, in partnership with E2-conjugating enzymes, selects protein substrates for ubiquitylation and their subsequent degradation [46,47,48]. Oxidative stress transforms DJ-1 (which has a key role in antioxidant activities as well as in directly inhibiting α-synuclein aggregation) into a more acidic isoform which translocates to the outer mitochondrial membrane with PINK1 (located on the inner membrane of mitochondria), regulating the function of mitochondria through phosphorylation of substrates. In neurodegeneration associated with PD, one or more of these pathways is impaired; losing parkin results in the accumulation of mitochondrial substrates, exposing mitochondria to increased stress, and proteasomal impairment amplifies this effect. A reduction in ATP exacerbates the proteasomal impairment, which leads to an increase in α-synuclein accumulation [43,49,50,51,52,53] and a further increase in neurodegeneration.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11030728
This entry is offline, you can click here to edit this entry!
