Osteoarthritis (OA) remains a prevalent disease affecting more than 20% of the global population, resulting in morbidity and lower quality of life for patients. The study of OA pathophysiology remains predominantly in animal models due to the complexities of mimicking the physiological environment surrounding the joint tissue. Development in microfluidic organ-on-chip (OoC) systems have demonstrated various techniques to mimic and modulate tissue physiological environments. Adaptations of these techniques have demonstrated success in capturing a joint tissue’s tissue physiology for studying the mechanism of OA.
- osteoarthritis
- cell microenvironment
- organ-on-chip
- disease models
1. Introduction
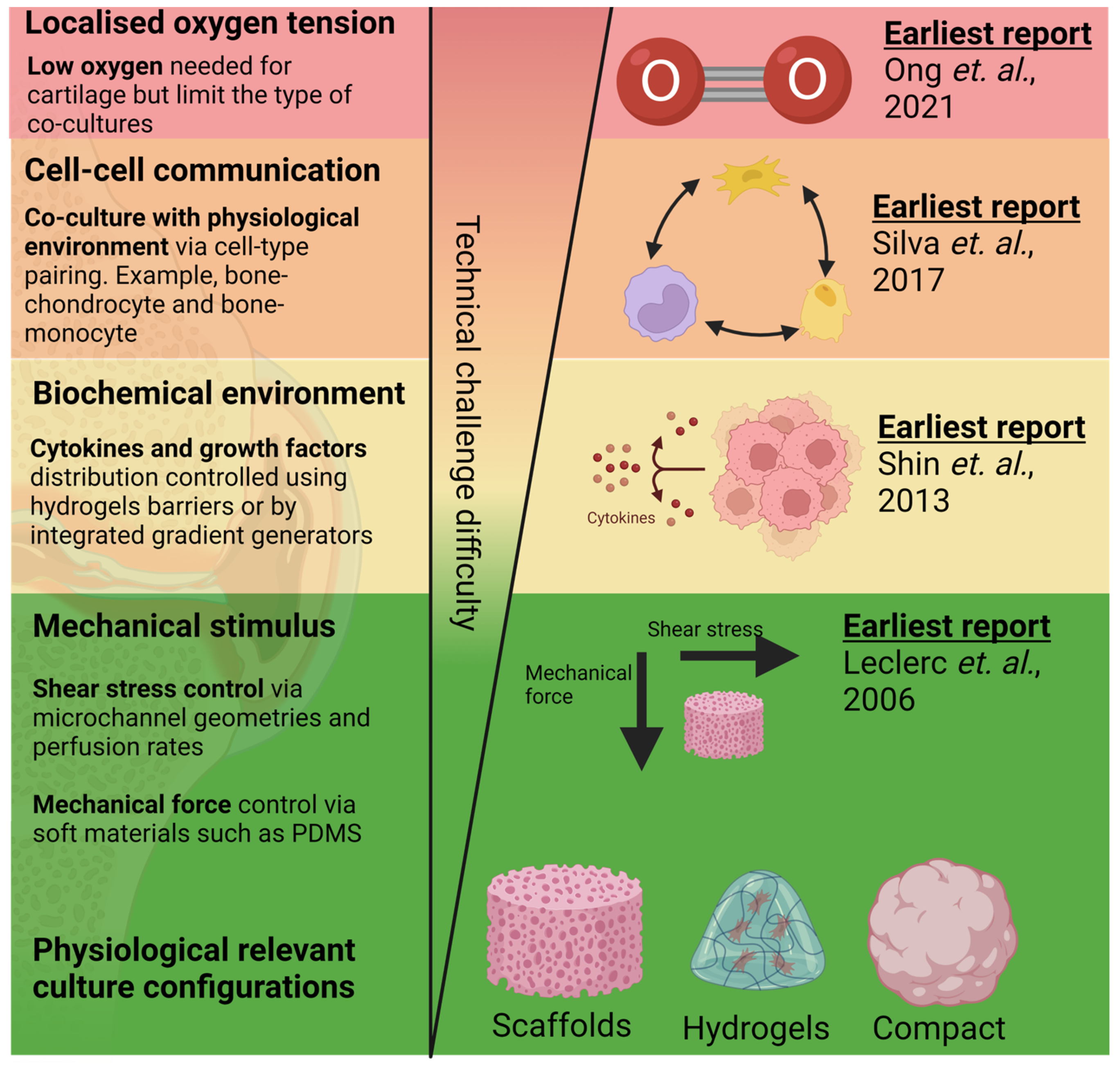
2. Bioengineering 3D Bone and Cartilage Tissues in OoCs

3. Application of Mechanical and Fluid-Induced Shear Stress
4. Mimicking Biochemical Environment/Chemical Gradient
5. Localised Control of Oxygen Tension
6. Mimicking Bone Cartilage Cross-Talk
| Co-Cultures | Tissue Culture Mode | Perfusion | Soluble Factor Controls | Ref |
|---|---|---|---|---|
| Bone|Cartilage | 3D hydrogel | Yes | No | [51] |
| Bone|Cartilage | 3D hydrogel | Yes | Yes | [65] |
| Bone|Cartilage | Scaffold | Yes | Yes | [66] |
| Bone|Neuron | 2D | No | Yes | [9] |
| Cartilage|Synoviocyte | 3D hydrogel | No | Yes | [31] |
| Bone|Monocytes | 3D hydrogel | Yes | No | [47][62] |
| Bone|Synoviocyte | 3D hydrogel | Yes | Yes | [21] |
This entry is adapted from the peer-reviewed paper 10.3390/cells12040579
References
- Leung, C.M.; De Haan, P.; Ronaldson-Bouchard, K.; Kim, G.A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Prim. 2022, 2, 33.
- Paggi, C.A.; Teixeira, L.M.; Le Gac, S.; Karperien, M. Joint-on-chip platforms: Entering a new era of in vitro models for arthritis. Nat. Rev. Rheumatol. 2022, 18, 217–231.
- Banh, L.; Cheung, K.K.; Chan, M.W.Y.; Young, E.W.; Viswanathan, S. Advances in organ-on-a-chip systems for modelling joint tissue and osteoarthritic diseases. Osteoarthr. Cartil. 2022, 30, 1050–1061.
- Jin, G.-Z.; Kim, H.-W. Effects of Type I Collagen Concentration in Hydrogel on the Growth and Phenotypic Expression of Rat Chondrocytes. Tissue Eng. Regen. Med. 2017, 14, 383–391.
- Leclerc, E.; David, B.; Griscom, L.; Lepioufle, B.; Fujii, T.; Layrolle, P.; Legallaisa, C. Study of osteoblastic cells in a microfluidic environment. Biomaterials 2006, 27, 586–595.
- Chao, P.G.; Tang, Z.; Angelini, E.; West, A.C.; Costa, K.D.; Hung, C.T. Dynamic osmotic loading of chondrocytes using a novel microfluidic device. J. Biomech. 2005, 38, 1273–1281.
- Shin, Y.; Han, S.; Jeon, J.S.; Yamamoto, K.; Zervantonakis, I.K.; Sudo, R.; Kamm, R.D.; Chung, S. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat. Protoc. 2012, 7, 1247–1259.
- Ong, L.J.Y.; Prasadam, I.; Lee, J.; Toh, Y.C. Microfluidic Patterning of Chondrocytes and Osteoblasts with Localised Oxygen Control. In Annual Micro Total Analysis Systems (MicroTAS) Conference; Chemical and Biological Microsystems Society (CBMS): Palm Spring, CA, USA, 2021.
- Silva, D.I.; dos Santos, B.P.; Leng, J.; Oliveira, H.; Amédée, J. Dorsal root ganglion neurons regulate the transcriptional and translational programs of osteoblast differentiation in a microfluidic platform. Cell Death Dis. 2017, 8, 1–14.
- Sun, L.; Wang, X.; Kaplan, D.L. A 3D cartilage—Inflammatory cell culture system for the modeling of human osteoarthritis. Biomaterials 2011, 32, 5581–5589.
- Rosser, J.; Bachmann, B.; Jordan, C.; Ribitsch, I.; Haltmayer, E.; Gueltekin, S.; Junttila, S.; Galik, B.; Gyenesei, A.; Haddadi, B.; et al. Microfluidic nutrient gradient–based three-dimensional chondrocyte culture-on-a-chip as an in vitro equine arthritis model. Mater. Today Bio 2019, 4, 100023.
- Choudhary, S.; Sun, Q.; Mannion, C.; Kissin, Y.; Zilberberg, J.; Lee, W.Y. Hypoxic three-dimensional cellular network construction replicates ex vivo the phenotype of primary human osteocytes. Tissue Eng. Part A 2018, 24, 458–468.
- Zhang, L.; Wen, C. Osteocyte Dysfunction in Joint Homeostasis and Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 6522.
- Paggi, C.A.; Venzac, B.; Karperien, M.; Leijten, J.C.; Le Gac, S. Monolithic microfluidic platform for exerting gradients of compression on cell-laden hydrogels, and application to a model of the articular cartilage. Sens. Actuators B Chem. 2020, 315, 127917.
- Bao, X.; Li, Z.; Liu, H.; Feng, K.; Yin, F.; Li, H.; Qin, J. Stimulation of chondrocytes and chondroinduced mesenchymal stem cells by osteoinduced mesenchymal stem cells under a fluid flow stimulus on an integrated microfluidic device. Mol. Med. Rep. 2017, 17, 2277–2288.
- Gottardi, R. Load-induced osteoarthritis on a chip. Nat. Biomed. Eng. 2019, 3, 502–503.
- Li, Y.; Fan, Q.; Jiang, Y.; Gong, F.; Xia, H. Effects of insulin-like growth factor 1 and basic fibroblast growth factor on the morphology and proliferation of chondrocytes embedded in Matrigel in a microfluidic platform. Exp. Ther. Med. 2017, 14, 2657–2663.
- Occhetta, P.; Mainardi, A.; Votta, E.; Vallmajo-Martin, Q.; Ehrbar, M.; Martin, I.; Barbero, A.; Rasponi, M. Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model. Nat. Biomed. Eng. 2019, 3, 545–557.
- Oliveira, I.M.; Carvalho, M.R.; Fernandes, D.C.; Abreu, C.M.; Maia, F.R.; Pereira, H.; Caballero, D.; Kundu, S.C.; Reis, R.L.; Oliveira, J.M. Modulation of inflammation by anti-TNF alpha mAb-dendrimer nanoparticles loaded in tyramine-modified gellan gum hydrogels in a cartilage-on-a-chip model. J. Mater. Chem. B 2021, 9, 4211–4218.
- Sahu, N.; Agarwal, P.; Grandi, F.; Bruschi, M.; Goodman, S.; Amanatullah, D.; Bhutani, N. Encapsulated Mesenchymal Stromal Cell Microbeads Promote Endogenous Regeneration of Osteoarthritic Cartilage Ex Vivo. Adv. Heal. Mater. 2021, 10, e2002118.
- Hou, H.W.; Menon, N.V.; Wee, S.N.; Li, K.H. Three-Dimensional (3D) Hydrogel Patterning in Microfluidic Vascular Models. U.S. Patent Application, 18 July 2019.
- Ma, H.-P.; Deng, X.; Chen, D.-Y.; Zhu, D.; Tong, J.-L.; Zhao, T.; Ma, J.-H.; Liu, Y.-Q. A microfluidic chip-based co-culture of fibroblast-like synoviocytes with osteoblasts and osteoclasts to test bone erosion and drug evaluation. R. Soc. Open Sci. 2018, 5, 180528.
- Hassan, S.; Cecen, B.; Peña-Garcia, R.; Marciano, F.; Miri, A.; Fattahi, A.; Karavasili, C.; Sebastian, S.; Zaidi, H.; Lobo, A. Survival and Proliferation under Severely Hypoxic Microenvironments Using Cell-Laden Oxygenating Hydrogels. J. Funct. Biomater. 2021, 12, 30.
- Shi, Y.; Ma, J.; Zhang, X.; Li, H.; Jiang, L.; Qin, J. Hypoxia combined with spheroid culture improves cartilage specific function in chondrocytes. Integr. Biol. 2015, 7, 289–297.
- Sieber, S.; Wirth, L.; Cavak, N.; Koenigsmark, M.; Marx, U.; Lauster, R.; Rosowski, M. Bone marrow-on-a-chip: Long-term culture of human haematopoietic stem cells in a three-dimensional microfluidic environment. J. Tissue Eng. Regen. Med. 2018, 12, 479–489.
- Bahmaee, H.; Owen, R.; Boyle, L.; Perrault, C.M.; Garcia-Granada, A.A.; Reilly, G.C.; Claeyssens, F. Design and Evaluation of an Osteogenesis-on-a-Chip Microfluidic Device Incorporating 3D Cell Culture. Front. Bioeng. Biotechnol. 2020, 8, 557111.
- Sun, Q.; Choudhary, S.; Mannion, C.; Kissin, Y.; Zilberberg, J.; Lee, W.Y. Ex vivo replication of phenotypic functions of osteocytes through biomimetic 3D bone tissue construction. Bone 2017, 106, 148–155.
- Szustak, M.; Gendaszewska-Darmach, E. Nanocellulose-Based Scaffolds for Chondrogenic Differentiation and Expansion. Front. Bioeng. Biotechnol. 2021, 9, 736213.
- Mekhileri, N.V.; Lim, K.S. Brown, G.C.J.; Mutreja, I.; Schon, B.S.; Hooper, G.; Woodfield, T.B.F. Automated 3D bioassembly of micro-tissues for biofabrication of hybrid tissue engineered constructs. Biofabrication 2018, 10, 024103.
- Watts, A.E.; Ackerman-Yost, J.C.; Nixon, A.J. A Comparison of Three-Dimensional Culture Systems to Evaluate In Vitro Chondrogenesis of Equine Bone Marrow-Derived Mesenchymal Stem Cells. Tissue Eng. Part A 2013, 19, 2275–2283.
- Rothbauer, M.; Byrne, R.A.; Schobesberger, S.; Calvo, I.O.; Fischer, A.; Reihs, E.I.; Spitz, S.; Bachmann, B.; Sevelda, F.; Holinka, J.; et al. Establishment of a human three-dimensional chip-based chondro-synovial coculture joint model for reciprocal cross talk studies in arthritis research. Lab A Chip 2021, 21, 4128–4143.
- Tian, K.; Zhong, W.; Zhang, Y.; Yin, B.; Zhang, W.; Liu, H. Microfluidics-based optimization of neuroleukin-mediated regulation of articular chondrocyte proliferation. Mol. Med. Rep. 2015, 13, 67–74.
- Wang, P.; Zhu, F.; Lee, N.; Konstantopoulos, K. Shear-induced Interleukin-6 Synthesis in Chondrocytes: Roles of e prostanoid (ep) 2 and ep3 in camp/protein kinase a- and pi3-k/akt-dependent nf-κb activation. J. Biol. Chem. 2010, 285, 24793–24804.
- Wang, P.; Guan, P.; Guo, C.; Zhu, F.; Konstantopoulos, K.; Wang, Z. Fluid shear stress-induced osteoarthritis: Roles of cyclooxygenase-2 and its metabolic products in inducing the expression of proinflammatory cytokines and matrix metalloproteinases. FASEB J. 2013, 27, 4664–4677.
- Smith, R.L.; Trindade, M.C.; Ikenoue, T.; Mohtai, M.; Das, P.; Carter, D.R.; Goodman, S.B.; Schurman, D.J. Effects of shear stress on articular chondrocyte metabolism. Biorheology 2000, 37, 95–107.
- Chang, S.-F.; Huang, K.-C.; Chang, H.-I.; Lee, K.-C.; Su, Y.-P.; Chen, C.-N. 2 dyn/cm2 shear force upregulates kruppel-like factor 4 expression in human chondrocytes to inhibit the interleukin-1β-activated nuclear factor-κB. J. Cell. Physiol. 2019, 234, 958–968.
- Yu, W.; Qu, H.; Hu, G.; Zhang, Q.; Song, K.; Guan, H.; Liu, T.; Qin, J. A Microfluidic-Based Multi-Shear Device for Investigating the Effects of Low Fluid-Induced Stresses on Osteoblasts. PLoS ONE 2014, 9, e89966.
- Yvanoff, C.; Willaert, R.G. Development of bone cell microarrays in microfluidic chips for studying osteocyte–osteoblast communication under fluid flow mechanical loading. Biofabrication 2022, 14, ac516e.
- Lieberthal, J.; Sambamurthy, N.; Scanzello, C. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1825–1834.
- Deszcz, I.; Lis-Nawara, A.; Grelewski, P.; Dragan, S.; Bar, J. Utility of direct 3D co-culture model for chondrogenic differentiation of mesenchymal stem cells on hyaluronan scaffold (Hyaff-11). Regen. Biomater. 2020, 7, 543–552.
- Miyaki, S.; Lotz, M.K. Extracellular vesicles in cartilage homeostasis and osteoarthritis. Curr. Opin. Rheumatol. 2018, 30, 129–135.
- Fan, X.; Wu, X.; Crawford, R.; Xiao, Y.; Prasadam, I. Micro, and Molecular. Changes of the Osteochondral Interface in Osteoarthritis Development. Front. Cell Dev. Biol. 2021, 9, 659654.
- Wu, X.; Crawford, R.; Xiao, Y.; Mao, X.; Prasadam, I. Osteoarthritic Subchondral Bone Release Exosomes That Promote Cartilage Degeneration. Cells 2021, 10, 251.
- Meng, Y.; Qiu, S.; Sun, L.; Zuo, J. Knockdown of exosome-mediated lnc-PVT1 alleviates lipopolysaccharide-induced osteoarthritis progression by mediating the HMGB1/TLR4/NF-κB pathway via miR-93-5p. Mol. Med. Rep. 2020, 22, 5313–5325.
- Irimia, D.; Geba, D.A.; Toner, M. Universal Microfluidic Gradient Generator. Anal. Chem. 2006, 78, 3472–3477.
- Chaudhary, R.; Campbell, K.; Tilbury, K.; Vanderby, R.; Block, W.F.; Kijowski, R.; Campagnola, P.J. Articular cartilage zonal differentiation via 3D Second-Harmonic Generation imaging microscopy. Connect. Tissue Res. 2015, 56, 76–86.
- Mondadori, C.; Palombella, S.; Salehi, S.; Talò, G.; Visone, R.; Rasponi, M.; Redaelli, A.; Sansone, V.; Moretti, M.; Lopa, S. Recapitulating monocyte extravasation to the synovium in an organotypic microfluidic model of the articular joint. Biofabrication 2021, 13, 045001.
- Su, C.; Chuah, Y.J.; Ong, H.B.; Tay, H.M.; Dalan, R.; Hou, H.W. A Facile and Scalable Hydrogel Patterning Method for Microfluidic 3D Cell Culture and Spheroid-in-Gel Culture Array. Biosensors 2021, 11, 509.
- Liu, H.-W.; Su, W.-T.; Liu, C.-Y.; Huang, C.-C. Highly Organized Porous Gelatin-Based Scaffold by Microfluidic 3D-Foaming Technology and Dynamic Culture for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 8449.
- Koo, H.-J.; Velev, O.D. Design and characterization of hydrogel-based microfluidic devices with biomimetic solute transport networks. Biomicrofluidics 2017, 11, 024104.
- Lin, H.; Lozito, T.; Alexander, P.; Gottardi, R.; Tuan, R.S. Stem cell-based microphysiological osteochondral system to model tissue response to interleukin-1beta. Mol. Pharm. 2014, 11, 2203–2212.
- Ju, Y.; Hu, Y.; Yang, P.; Xie, X.; Fang, B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater. Today Bio 2023, 18, 522.
- Bondeson, J.; Wainwright, S.D.; Lauder, S.; Amos, N.; Hughes, C.E. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res. Ther. 2006, 8, R187.
- Brennan, M.D.; Rexius-Hall, M.L.; Elgass, L.J.; Eddington, D.T. Oxygen control with microfluidics. Lab Chip 2014, 14, 4305–4318.
- Rankin, E.B.; Giaccia, A.; Schipani, E. A Central Role for Hypoxic Signaling in Cartilage, Bone, and Hematopoiesis. Curr. Osteoporos. Rep. 2011, 9, 6–52.
- Lin, J.-L.; Wang, M.J.; Lee, D.; Liang, C.-C.; Lin, S. Hypoxia-inducible factor-1α regulates matrix metalloproteinase-1 activity in human bone marrow-derived mesenchymal stem cells. FEBS Lett. 2008, 582, 2615–2619.
- Tuerlings, M.; Boone, I.; Amirabadi, H.E.; Vis, M.; Suchiman, E.; van der Linden, E.; Hofmann, S.; Nelissen, R.; Toonder, J.D.; Ramos, Y.; et al. Capturing Essential Physiological Aspects of Interacting Cartilage and Bone Tissue with Osteoarthritis Pathophysiology: A Human Osteochondral Unit-on-a-Chip Model. Adv. Mater. Technol. 2022, 7, 1310.
- Chen, Y.-A.; King, A.D.; Shih, H.-C.; Peng, C.-C.; Wu, C.-Y.; Liao, W.-H.; Tung, Y.-C. Generation of oxygen gradients in microfluidic devices for cell culture using spatially confined chemical reactions. Lab Chip 2011, 11, 3626–3633.
- Maschmeyer, I.; Lorenz, A.; Schimek, K.; Hasenberg, T.; Ramme, A.; Hübner, J. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab A Chip 2015, 15, 2688–2699.
- Materne, E.-M.; Ramme, A.P.; Terrasso, A.P.; Serra, M.; Alves, P.M.; Brito, C.; Sakharov, D.A.; Tonevitsky, A.G.; Lauster, R.; Marx, U. A multi-organ chip co-culture of neurospheres and liver equivalents for long-term substance testing. J. Biotechnol. 2015, 205, 36–46.
- Kim, G.-J.; Lee, K.-J.; Choi, J.-W.; An, J. Drug Evaluation Based on a Multi-Channel Cell Chip with a Horizontal Co-Culture. Int. J. Mol. Sci. 2021, 22, 6997.
- Wei, C.-W.; Cheng, J.-Y.; Young, T.-H. Elucidating in vitro cell-cell interaction using a microfluidic coculture system. Biomed. Microdevices 2006, 8, 65–71.
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348.
- Conaghan, P.G.; D’Agostino, M.; Le Bars, M.; Baron, G.; Schmidely, N.; Wakefield, R. Clinical and ultrasonographic predictors of joint replacement for knee osteoarthritis: Results from a large, 3-year, prospective EULAR study. Ann. Rheum. Dis. 2010, 69, 644–647.
- Lin, Z.; Li, Z.; Li, E.N.; Li, X.; Del Duke, C.J.; Shen, H.; Hao, T.; O’Donnell, B.; Bunnell, B.A.; Goodman, S.B.; et al. Osteochondral Tissue Chip Derived From iPSCs: Modeling OA Pathologies and Testing Drugs. Front. Bioeng. Biotechnol. 2019, 7, 411.
- Tuerlings, M.; Boone, I.; Amirabadi, H.E.; Toonder, J.D.; Ramos, Y.; Meulenbelt, I. Development of a human osteochondral construct on a microfluidic chip-to advance functional studies of osteoarthritis risk genes. Osteoarthr. Cartil. 2021, 29, S108–S109.
- Han, D.; Fang, Y.; Tan, X.; Jiang, H.; Gong, X.; Wang, X.; Hong, W.; Tu, J.; Wei, W. The emerging role of fibroblast-like synoviocytes-mediated synovitis in osteoarthritis: An update. J. Cell. Mol. Med. 2020, 24, 9518–9532.
- Chou, C.-H.; Jain, V.; Gibson, J.; Attarian, D.E.; Haraden, C.A.; Yohn, C.B.; Laberge, R.-M.; Gregory, S.; Kraus, V.B. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep. 2020, 10, 1–9.
- Nanus, E.D.; Badoume, A.; Wijesinghe, S.N.; Halsey, A.M.; Hurley, P.; Ahmed, Z.; Botchu, R.; Davis Lindsay, A.M.; Jones, S.W. Synovial tissue from sites of joint pain in knee osteoarthritis patients exhibits a differential phenotype with distinct fibroblast subsets. Ebiomedicine 2021, 72, 3618.
- Hussain, A.; Tebyaniyan, H.; Khayatan, D. The Role of Epigenetic in Dental and Oral Regenerative Medicine by Different Types of Dental Stem Cells: A Comprehensive Overview. Stem Cells Int. 2022, 2022, 1–15.
- Xiang, X.-N.; Zhu, S.-Y.; He, H.-C.; Yu, X.; Xu, Y.; He, C.-Q. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res. Ther. 2022, 13, 1–20.
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 1–13.
- Ashraf, S.; Walsh, D.A. Angiogenesis in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 573–580.
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81.
- Ko, J.; Lee, Y.; Lee, S.; Lee, S.; Jeon, N.L. Human Ocular Angiogenesis-Inspired Vascular Models on an Injection-Molded Microfluidic Chip. Adv. Heal. Mater. 2019, 8, e1900328.
