The heavy metals found in soil and sediments are lead (Pb), chrome (Cr), mercury (Hg), zinc (Zn), copper (Cu), nickel (Ni), and cobalt (Co), and must be made to form stable complexes with anionic biosurfactants for precipitation and removal from the soil [
22]. Washing with a surfactant solution can be performed for the remediation of soils contaminated by heavy metals. Moreover, the biosurfactant can be reused several times for the cleaning of contaminated soil, also enabling the reuse of the metals in the battery industry [
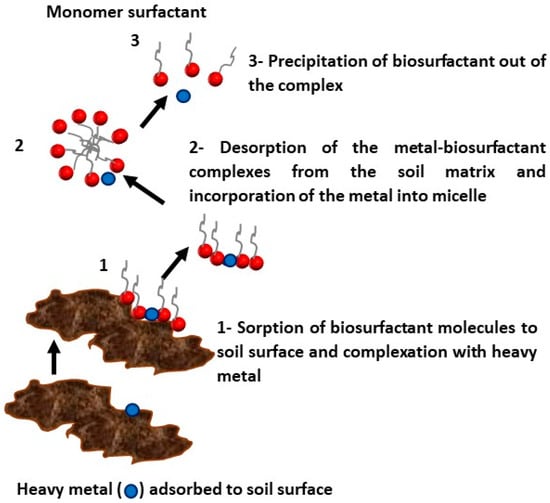
54]. Anionic surfactants form complexes with metals by ionic bonds, which are normally stronger than the bonds between the metal and soil. The interaction between the biosurfactant and the metal is expected to be stronger than the interaction between the metal and the humic acids present in the soil, which act as chelating agents. The reduction in surface tension enables the desorption of the metal–surfactant complex from the soil matrix. The mechanism of removal by ionic surfactants occurs through the sorption of the surfactant to the soil surface, followed by complexation with the metal, detachment of the metal from the soil, and its uptake by micelles of the surfactant, as illustrated in
Figure 1. Electrostatic interactions maintain the heavy metal imprisoned in the micelles, enabling subsequent recovery with the use of membrane separation methods [
43].
Rocha Júnior et al. [
54] showed that, when the concentration is high, a biosurfactant takes the place of metals by reacting with soil particles, as demonstrated with an anionic biosurfactant produced by
C. tropicalis applied at ½ the CMC, the CMC, and 2 × the CMC for the removal of Zn, Pb, and Cu from sand. A higher removal rate was found with the increase in the concentration of the biosurfactant. While the biosurfactant was effective in the static test, the dynamic test enabled the greater removal of these contaminants. The kinetic test determined that 30 min were sufficient for the biosurfactant to remove the heavy metals. Moreover, recycling tests demonstrated that the first washing removed nearly all the lead and copper from the soil, whereas the second washing removed 9% of these contaminants [
54]. Biosurfactant-assisted phytoremediation enabled the following heavy metal removal rates: 41% for Ni, 30% for Cr, 29% for Pb, and 20% for zinc [
56]. An anionic biosurfactant produced by
C. sphaerica UCP0995 was tested in different combinations with NaOH and HCl for removing heavy metals from soil, with Fe, Zn, and Pb removal rates of 95, 90, and 79%, respectively [
57]. The anionic crude biosurfactant produced by the yeast
C. guilliemondii UCP 0992 achieved Fe, Zn, and Pb removal rates of 89.3, 98.8, and 89.1%, respectively [
58]. Removal rates of 95 and 52% were found for Cd and Pb, respectively, with the use of a sophorolipid produced by
S. bombicola [
59]. Biosurfactants can also be used to inhibit the corrosion of oil pipelines, as cations such as Na
+ in the environment tend to take the place of the metallic cations of the pipe, whereas biosurfactants tend to adsorb to the surface of the metal, inhibiting corrosion [
12].
3. Application of Biosurfactants for Bioremediation of Marine Environment
Oceans are the home to the largest oil reserves and, hence, the location of the largest oil spills. The transport and use of oil leads to the generation of oily effluents in the marine environment as well as the emission of gaseous pollutants [
12]. Conventional remediation is classified as (i) physical or mechanical methods employing floating barriers known as booms and skimmers to contain and suck up the spilled oil and hydrophobic absorbents that repel water and capture the oil; (ii) biological and (iii) chemical, the latter of which includes on-site burning, the use of solidifiers, or gelling agents that transform the oil into a solid compound and dispersants, which are composed of surfactants, organic solvents, stabilizers, and other additives that break up the oil into emulsified droplets (oil-in-water emulsion) and accelerate the natural bioremediation process [
60,
61,
62].
Although efficient at the remediation of oil spills in the marine environment, chemical dispersants do not biodegrade easily and are toxic to aquatic organisms, underscoring the need to replace these products with effective, biodegradable, ecologically friendly dispersants.
Biosurfactants have considerable stability when exposed to different environmental conditions. Microbial surfactants can maintain their surface-active properties in the presence of 2–10% salinity, a pH range of 3–12, and high temperatures (e.g., 70 °C), making these natural compounds viable for application in extreme environments with high salinity, such as the Dead Sea, where archeobacteria and halophilic bacteria produce polysaccharides and assist in the use of biosurfactants [
19].
As in the remediation of oil-contaminated soil, the biosurfactant-mediated biodegradation of hydrocarbons occurs via two mechanisms. One involves the enhancement of the availability of the hydrophobic substrate to microorganisms by reducing the medium surface tension and reducing the interfacial tension between the cell wall and hydrocarbon molecules. The other mechanism involves the interaction between the biosurfactant and the cell surface, leading to alterations in the membrane, facilitating hydrocarbon adherence (increase in hydrophobicity), and reducing lipopolysaccharides of the cell wall without harming the membrane [
4]. Biosurfactants block the formation of hydrogen bridges and allow hydrophobic–hydrophilic interactions, promoting molecular rearrangements, reducing the medium surface tension, increasing its surface area, and favoring bioavailability and, consequently, biodegradability [
23].
An analysis of dispersion capacity revealed that a biosurfactant with a low CMC was effective; however, the dispersant at a concentration 1.5 times above the CMC was ineffective for biodegradation in marine remediation [
63]. Above the optimal concentration, biosurfactants cover the biosurfactant–hydrocarbon complexes, either impeding the access of microorganisms to the hydrocarbons or becoming the substrate used by the microorganisms [
64,
65].
Several studies report the use of biosurfactants in marine environments.
P. aeruginosa UCP 0992 grown in corn steep liquor and waste vegetable oil in a bioreactor produced a biosurfactant that reduced the surface tension of the medium to 26.5 mN/m, with a yield of 26 g/L. The biosurfactant was capable of removing 90% and 80% of motor oil adsorbed to sand in kinetic and static tests, respectively. Experiments revealed degradation rates higher than 90% for the oil in the presence of the bacterium and biosurfactant in seawater samples over a period of 75 days, demonstrating the potential of the biosurfactant in the oil decontamination process in a marine environment [
50]. Experiments with a marine indicator demonstrated the stability and non-toxicity of the biosurfactant produced by
C. bombicola URM 3718 cultivated in industrial waste and formulated with the addition of potassium sorbate. Tests involving different pH and temperature values in the presence of salt demonstrated that a commercial biosurfactant has potential applications in the environmental field [
66]. A biosurfactant produced by the yeast
C. lipolytica promoted the degradation of a petroleum by autochthonous microorganisms from seawater [
67]. Bacteria isolated from contaminated seawater were studied for the production of biosurfactants and subsequent use in the remediation of marine environments. Genetic sequences revealed promising isolates from the same species (
B. cereus). The BCS0 strain cultivated in a mineral medium with 2% waste fry oil was the best producer of surfactant, being able to reduce the surface tension to 27 mN/m. The biosurfactant remained stable in a wide range of pH and temperatures and in the presence of salinity, enhancing the degradation of motor oil in seawater up to 96% in 27 days. The biosurfactant also exhibited considerable oil displacement capacity [
68]. The compound was characterized as a non-toxic lipopeptide, as survival rates for the fish
Poecilia vivipara and the bivalve
Anomalocardia brasiliana were higher than 90% and 55%, respectively. The biosurfactant also promoted the growth of autochthonous microorganisms in seawater [
69]. Studying a biosurfactant produced by the same microorganism (
B. cereus), Ostendorf et al. [
70] found that the biomolecule was capable of removing 90% of oil adsorbed to marine rock and dispersed 70% of the contaminant in seawater.
The biosurfactant produced by the bacterium
P. cepacia CCT6659 cultivated in industrial waste products and formulated with a food conservative had low toxicity to a plant and marine aquatic species and was able to remove about 70% and 84.50% of hydrophobic contaminants adsorbed to sand and marine rock, respectively. The biosurfactant also dispersed 96% of oil in seawater and increased the degradation of the oil by 70% in bioremediation experiments performed in flasks containing seawater [
71]. The oil dispersion capacity of a biosurfactant from
C. lipolytica was tested using a test developed by the US Environmental Protection Agency as a protocol for classifying oil spill dispersants (baffled flask test) [
67]. The authors reported a dispersion rate ranging from 50 to 100%, depending on the concentration of the biosurfactant and its form of application (crude or isolated).
Binary systems involving the combination of biosurfactants and chemical surfactants have also been developed for the less-toxic treatment of oil spills. The system formed by ethanediyl-1,3-bis(dodecyl dimethyl ammonium bromide) (cationic surfactant) and surfactin from
B. subtilis successfully reduced the interfacial tension of crude oil [
72]. A binary mixture of the ionic surfactant choline laurate and a sophorolipid produced by
S. bombicola proved non-toxic to fish and achieved dispersion rates greater than 80% [
73].
4. Application of Biosurfactants for Oil Recovery from Reservoirs
Crude oil is a mixture of water, gas, and the organic fraction together with appropriate thermochemical conditions found in sedimentary rock [
12]. The process used to extract crude oil involves three steps. The first and second steps are natural pressure and induced pressure (injection of water and gas). These steps extract approximately 40% of the trapped oil. The third step is the recovery of the residual oil. Enhanced Oil Recovery (EOR) involves the use of thermal methods (injection of hot water and carbon dioxide [CO
2]), non-thermal methods (flooding with solvents and chemical surfactants), and biological methods, although removing this oil from rock is difficult [
12].
Synthetic surfactants in this process require capital and cause environmental pollution [
74]. In contrast, the use of biosurfactants in EOR can provide favorable conditions and solve problems associated with environmental pollution [
10]. Microbial Enhanced Oil Recovery (MEOR) involves the use of microorganisms or their metabolic products to retrieve the residual oil [
75]. This procedure generally involves the injection of biosurfactant producer microorganisms, followed by the injection of nutrients into the reservoir (in situ biosurfactant production). Ex situ biosurfactant production can also be performed in industrial bioreactors for the subsequent direct injection of these compounds into the reservoir using CO
2. The emulsifiers/surfactants produced by microorganisms lower the surface tension, resulting in the release of the trapped oil. Biosurfactants alter the wettability of the injected CO
2 and the interfacial behavior of CO
2-brine-rock, increasing the flushing efficiency of the injected fluid and CO
2, resulting in the recovery of the oil [
12,
74,
76].
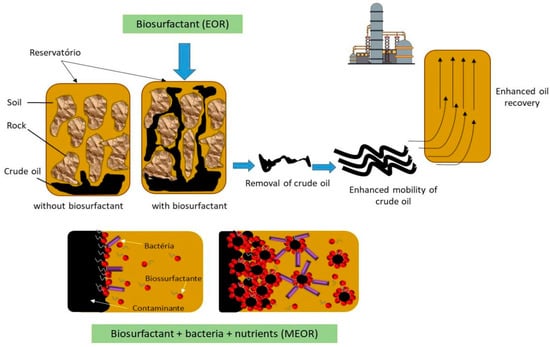
Figure 2 shows the effect of biosurfactants on oil/rock and oil/water interfaces, decreasing the capillary forces that impede the oil from moving through the pores of the rock during the EOR process. Biosurfactants also form an emulsion at the oil/water interface that stabilizes the oil desorbed in water and allows its removal with the injection water [
23].
Figure 2. Enhanced Oil Recovery (EOR) and Microbial Enhanced Oil Recovery (MEOR) using biosurfactants (created by the authors).
Another method consists of the injection of only nutrients to stimulate the growth of indigenous biosurfactant-producing microorganisms in reservoirs. With all mechanisms, the biosurfactant lowers the surface tension of the water and the interfacial tension between the oil and rock, enabling the removal of the hydrocarbons [
12]. Laboratory experiments involving the use of crude biosurfactants in sand-pack columns reported oil recovery rates of 15% [
77], 30% [
49], 80% [
50], and 65–80% [
78], demonstrating the viability of these ecological agents in EOR and MEOR processes.
One study reported the isolation of biosurfactant-producing strains capable of degrading crude oil isolated from the reservoir of an oil field in Assam, India.
B. Tequilensis exhibited good oil degradation properties and biosurfactant production, which was optimized ex situ. The crude biosurfactant was identified as a surfactin, achieving an interfacial tension of 0.32 mN/m and removing 80% of the oil during washing procedures [
79].
MEOR was evaluated by an economic model that simulates oil recovery operations using a bench-scale project [
18]. A biosurfactant from
B. methylotrophicus cultivated in industrial waste was able to remove 63% of oil adsorbed to sand and 25% adsorbed to sandy soil [
80]. Contaminant removal rates are affected by the type and concentration of the biosurfactant, the affinity with the contaminant, interactions with acid or alkaline additives, and soil characteristics, as described by Adrion et al. [
81].
5. Application of Biosurfactants in Transport of Crude Oil through Pipelines
The long-distance transport of viscous crude oil from the extraction field to strategic destinations poses a problem due to the high content of asphaltenes and paraffins, which can cause obstructions of the pipeline [
87]. This problem could be minimized with the use of larger pipes and stronger pumps to increase the pressure, the heating, or the use of toluene and xylene to dissolve the sludge. Despite the efficiency of these solutions, they are expensive and release toxic substances into the environment [
10].
Paraffin wax deposition from crude oils at a low temperature is one of the serious problems in the petroleum transportation industry. As one of the low-temperature resistance properties of crude oil, the wax appearance temperature (WAT) is the temperature at which visible crystallization occurs. When the temperature falls below the WAT, the waxes tend to separate from the oil, crystallizing to form interlocking network structures, thereby entrapping the remaining liquid fuel in the structures [
88].
Wax build-up that occurs in conventional middle and light crude oils is also a complicated and very costly problem for the petroleum industry. One viable solution of this problem is the employment of polymeric additives to improve the flow behavior of crude oil at low temperatures. Polymeric additives act as crystal modifiers, flow improvers, or pour-point depressants (PPDs), and are able to build crystals and modify the crystal morphology and growth characteristics into wax, reducing the tendency to interconnect into three-dimensional networks [
88].
In this scenario, biosurfactants with emulsifying properties are indicated, as the formation of emulsions is essential to transporting oil. Unlike a water-in-oil emulsion, the stabilization of an oil-in-water emulsion requires the separation of the phases once the crude oil transported through the pipeline reaches its destination (refinery). The reduction in tension is not a concern in such cases and the surfactant should have a high HLB. Thus, biosurfactants with a high HLB (bioemulsifiers) are highly useful to increase the mobility of oil. Such cases involve the use of biosurfactants with a high molar mass, such as alasan, emulsan, and polymeric biosurfactants.
The bacterial surfactant Emulsan has many reactive groups that enable the molecule to secure firmly to oil droplets, avoiding the coalescence of these droplets by forming a barrier [
18]. An emulsan produced by
Acinetobacter sp. was used to reduce the viscosity of oil during transportation. Upon arriving at the destination, treatment can be performed with enzymes or demulsifiers to remove the bioemulsifiers from the emulsion [
89]. Working with emulsifiers requires producing large volumes of this type of surfactant for the transportation of oil;however, it could avoid the need for a new pipeline [
10]. Two bacterial biosurfactants were successfully added as a mixture or individually to crude oil to reduce the pour point [
88]. A biobased surfactant obtained by enzymatic synthesis was tested for wax deposition inhibition. A maximum viscosity reduction of 70% and a drag reduction of 40% for light crude oil flows in pipelines were obtained with the surfactant additive at a concentration of 100 mg/L. Furthermore, a successful field application of the drag-reducing surfactant in a light crude oil pipeline in Daqing Oilfield was demonstrated [
90].