Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Pulsed laser ablation in liquid (PLAL) is a physical and top-down approach used to fabricate nanoparticles (NPs). NPs have better physicochemical properties than their bulk counterparts.
- laser ablation
- bimetallic nanoparticles
- green manufacturing
1. Overview
Pulsed laser ablation in liquid (PLAL) is a top-down and physical NP synthesis method that has been around for more than two decades, yet its popularity and usage in the industry are still low. PLAL involves laser processing of a solid target under a liquid (usually DI water) to produce a colloid, as shown in Figure 1. Various parameters (laser pulse width, laser fluence, repetition rate, type of liquid medium, etc.) need to be controlled to produce a specific NP size distribution, yield, and shape [1][2][3][4][5]. Due to their advanced physicochemical properties, the resulting NPs have versatile uses in a wide range of fields, including enhancing the electrical properties during the additive manufacturing of polymers [5][6] and in the production of flexible electronics [7][8]. PLAL has been used to fabricate various types of NPs, including C [3][9], Ti [10], and Ag [11]. The PLAL process can be divided into three main stages, as shown in Figure 1. Stage 1 involves the interaction of the laser with the liquid medium, which incorporates the formation of electron clouds through photon absorption by liquid medium molecules. Each liquid medium will interact with the laser differently, resulting in different outputs. Stage 2 of PLAL involves the interaction of the laser with the target. This stage involves the formation of the plasma plume, the involvement of electron clouds in the ablation process, the formation of cavitation bubbles, and the formation of nuclei. Stage 3 of PLAL involves the growth of nuclei into NPs inside the cavitation bubble, the involvement of ions from the liquid medium during the nucleation process, the growth of NPs, collisional events between NPs, agglomeration events, and NP ageing. There are a great deal of physicochemical equations governing the PLAL process, and it is difficult to gather them all in one simulation. Most publications simulate PLAL in stages (Stages 1,2, or 3), making assumptions for the other stages due to the complexity and sensitivity of the process to the inputs. A reader who is interested in the equations governing the PLAL process can find Stage 1 equations in the previous publication here [12]; Stage 2 equations were covered by Povarnitsyn et al. [13] and Ibrahimkutty et al. [14]; and Stage 3 equations were covered by Dell’Aglio et al. [15] and Taccogna et al. [16].
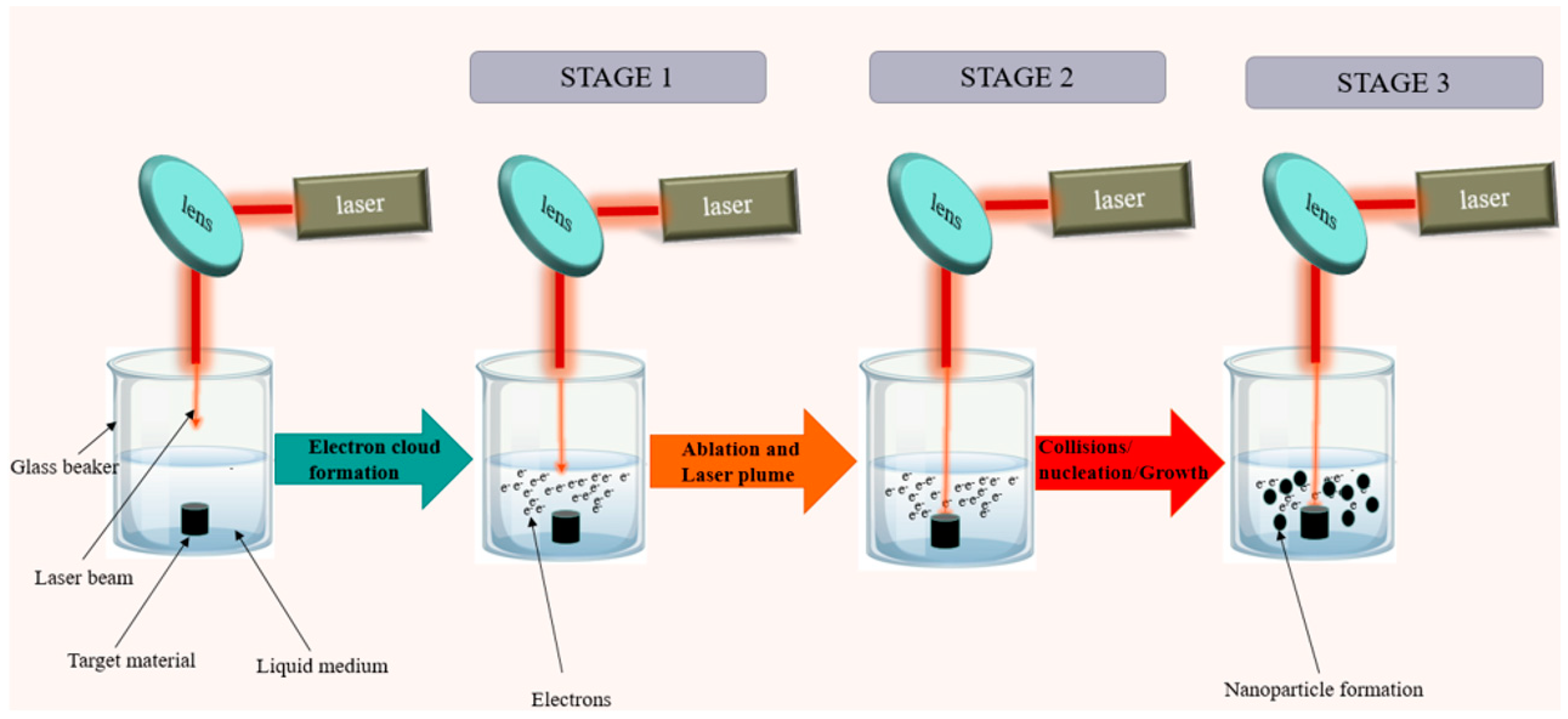
Figure 1. Illustration of the PLAL process in batch mode showing the 3 main stages of PLAL [12].
Another interesting and highly researched area is the application of metallic nanoparticles made from noble metals, such as Ag, Pt, Pd, and Au, in catalysis. Catalysts are used in more than 80% of all manufacturing processes, and heterogeneous catalysts are involved in 90% of those [17][18]. Catalysis is involved in the refining of petroleum, fertilizer synthesis, polymer synthesis, and in catalytic convertors, to mention a few examples. NPs have gained attention in catalysis owing to their large surface-area-to-volume ratio, high surface reactivity, and high optical absorbance. The high surface reactivity is by far the most important property of NPs in catalysis applications, and the high surface area ranks second. During catalysis using NPs, the active sites on the surfaces of the NPs react with the substrate to catalyse the reaction. Therefore, uncapped, ligand-free or surfactant-free NPs are preferred over capped ones owing to the higher number of exposed reaction sites and a high number of free electrons on the surface of the NPs [17][18][19][20][21]. To that end, PLAL is a favourable and ideal method of NP synthesis for catalysis applications. PLAL-synthesised NPs are clean, uncapped, and surfactant-free, which makes their surfaces highly reactive. Surfactants are often added to reduce NP agglomeration, but this is not required in catalysis, whereby the reactivity is of more importance than the agglomeration [18][21].
2. Modes of Pulsed Laser Ablation in Liquid (PLAL)
There are currently two main modes (experimental set-up) of PLAL, namely batch mode (Figure 2a) and flow mode (Figure 2b). The two modes are distinguished by the motion or lack thereof of the liquid environment during ablation. In batch mode, the liquid medium is stationary, while in flow mode, the liquid medium is flowing or agitated in some way. Both methods have their merits, with batch mode being used mainly for research purposes, while flow mode was recently introduced in an attempt to increase the NP yield towards the industrial usage of PLAL. Certainly, the flow mode PLAL has drastically increased the NP yield/colloidal density due to its ability to instantly carry the recently ablated material and bubbles away from the ablation zone [22][23], thereby increasing NP yield.

Figure 2. The two modes of pulsed laser ablation in liquid (PLAL): (a) batch mode and (b) flow mode.
For instance, a 380% increase in ablation efficiency was observed when ablating Ag NPs via flow mode instead of batch mode [24]. The batch mode set-up is shown in Figure 2a, whereby PLAL is conducted in a beaker or a vial for a certain ablation time, and afterwards, the target is removed and the colloid is collected, characterised, and used for various purposes. The volume of the liquid is limited to the size of the ablation container, which is one of the limitations of batch mode. Conversely, in flow mode PLAL, the liquid flows/is agitated at a controlled speed and can be collected in a different vessel. This enables a high volume of the colloid to be collected in one continuous process. Another advantage of flow mode is the increased NP yield per hour due to the reduced shielding effects by the flowing liquid. Shielding effects involve the laser plume, cavitation bubble, and nanoparticles being in the path of the laser, thereby shielding the target surface from absorbing incoming laser photons, resulting in reduced NP yield. One of the disadvantages of flow mode is the need for additional equipment, such as pumps, flow cells, and automation devices, and the additional energy consumption to run these. The additional apparatus in flow mode increases the risk of NP contamination (from previous experiments) and the loss of NPs as they pass through various instruments, for example, the pump and tubes. Batch mode PLAL is fast at producing results, adaptable, easy to set up, and does not require additional equipment, such as pumps, flow cells, and automation apparatus. Additionally, the risk of contamination and downtime is reduced in batch mode PLAL due to the reduced number of components.
Other innovative techniques have been implemented in PLAL technology in the pursuit of increasing the yield and controlling the NP size and shape. Electric fields have been used during PLAL, a process now termed electric-field-assisted laser ablation in liquid (EFLAL) [25]. Magnetic fields have also been incorporated, resulting in magnetic field-assisted laser ablation in liquid (MFLAL) [26][27]. It has been reported that electric fields increase NP yield [1]. It has been reported that the size of Bi2O3 NPs obtained via PLAL increases with the application of an electric field [28]. Furthermore, PLAL has been accompanied by additional processing techniques, namely laser fragmentation in liquid (LFL), laser melting in liquid (LML), and laser photoexcitation in liquid (LPL) [5][29][30]. LFL, LML, and LPL are conducted after PLAL when the NPs are synthesised and the target is removed. These processes are conducted to modify the synthesised NPs. LFL, LML, and LPL are conducted to reduce the NP size, increase the NP size/reshape NP, and modify the surface chemistry (e.g., oxidation/reduction), respectively. Not much attention has been paid to these additional techniques, which highlight a gap in the literature that can be explored.
In another publication, NP ageing experiments were conducted on Mg NPs that were synthesised via PLAL in isopropanol alcohol (IPA). The Mg NPs increased in NP mean size from 50 to 200 nm after 9 months of storage at room temperature, as shown in Figure 3a. The colour of the colloid changed from yellow to grey after 9 months, which was caused by the increase in NP size, as shown in Figure 3a (top). The aged colloid was laser processed again for 30 minutes, and the NP mean size was reduced to 34 nm due to NP fragmentation. The NP colloid original yellow colour was restored, which further demonstrated the dependence of the colloidal colour on the NP mean size. The aforementioned experiment suggests that PLAL-synthesised colloids have a specific shelf-life and may require more processing to restore the NP size.
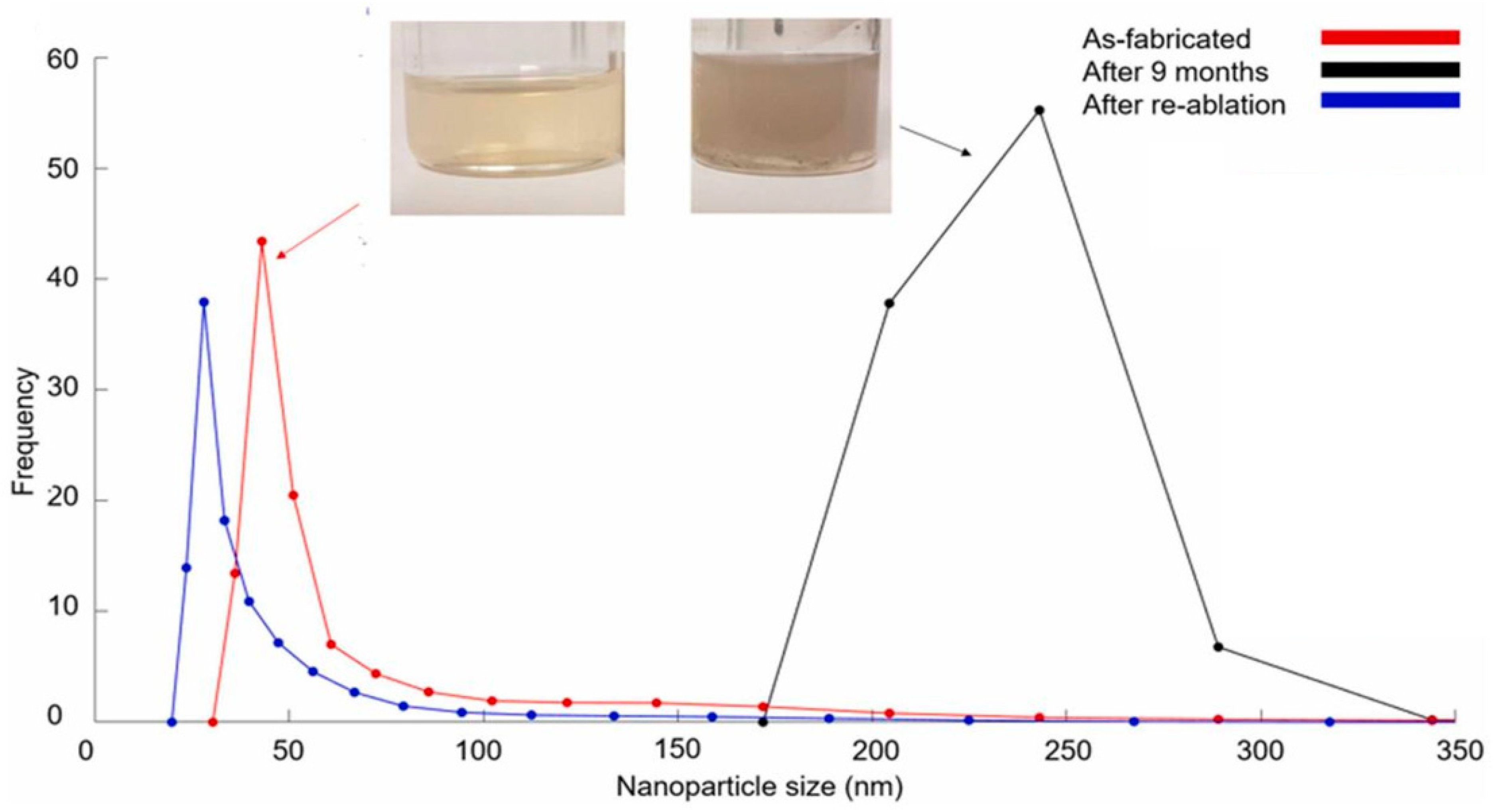
Figure 3. (a) Laser fragmentation in liquid effect; (b) dried magnesium nanoparticles in a glass vial [30].
This entry is adapted from the peer-reviewed paper 10.3390/cryst13020253
References
- Fazio, E.; Gökce, B.; De Giacomo, A.; Meneghetti, M.; Compagnini, G.; Tommasini, M.; Waag, F.; Lucotti, A.; Zanchi, C.G.; Ossi, P.M.; et al. Nanoparticles engineering by pulsed laser ablation in liquids: Concepts and applications. Nanomaterials 2020, 10, 2317.
- Al-Hamaoy, A.; Chikarakara, E.; Jawad, H.; Gupta, K.; Kumar, D.; Rao, M.S.R.; Krishnamurthy, S.; Morshed, M.; Fox, E.; Brougham, D.; et al. Liquid Phase—Pulsed Laser Ablation: A route to fabricate different carbon nanostructures. Appl. Surf. Sci. 2014, 302, 141–144.
- Nyabadza, A.; Vázquez, M.; Fitzpatrick, B.; Brabazon, D. Effect of liquid medium and laser processing parameters on the fabrication of carbon nanoparticles via pulsed laser ablation in liquid towards paper electronics. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128151.
- Nyabadza, A.; Vázquez, M.; Coyle, S.; Fitzpatrick, B.; Brabazon, D. Magnesium Nanoparticle Synthesis from Powders via Pulsed Laser Ablation in Liquid for Nanocolloid Production. Appl. Sci. 2021, 11, 10974.
- Amans, D.; Cai, W.; Barcikowski, S.; Info, A.; Amans, D.; Cai, W.; Barcikowski, S. Status and demand of research to bring laser generation of nanoparticles in liquids to maturity. Appl. Surf. Sci. 2019, 488, 445–454.
- Nyabadza, A.; Kane, J.; Sreenilayam, S.; Vázquez, M.; Brabazon, D.; Sreenilayam, S.; Brabazon, D. Multi-Material Production of 4D Shape Memory Polymer Composites. In Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 879–894.
- Nyabadza, A.; Vázquez, M.; Coyle, S.; Fitzpatrick, B.; Brabazon, D. Review of materials and fabrication methods for flexible nano and micro-scale physical and chemical property sensors. Appl. Sci. 2021, 11, 8563.
- Kanitz, A.; Kalus, M.R.; Gurevich, E.L.; Ostendorf, A.; Barcikowski, S.; Amans, D. Review on experimental and theoretical investigations of the early stage, femtoseconds to microseconds processes during laser ablation in liquid-phase for the synthesis of colloidal nanoparticles. Plasma Sources Sci. Technol. 2019, 28, 103001.
- Bagga, K.; McCann, R.; Wang, M.; Stalcup, A.; Vázquez, M.; Brabazon, D. Laser assisted synthesis of carbon nanoparticles with controlled viscosities for printing applications. J. Colloid Interface Sci. 2015, 447, 263–268.
- Jasbi, N.E.; Dorranian, D. Effect of aging on the properties of TiO2 nanoparticle. J. Theor. Appl. Phys. 2016, 10, 157–161.
- McCarthy, É.; Sreenilayam, S.P.; Ronan, O.; Ayub, H.; McCann, R.; McKeon, L.; Fleischer, K.; Nicolosi, V.; Brabazon, D. Silver nanocolloid generation using dynamic Laser Ablation Synthesis in Solution system and drop-casting. Nano-Struct. Nano-Objects 2022, 29, 100841.
- Nyabadza, A.; Vázquez, M.; Brabazon, D. Modelling of Pulsed Laser Ablation in Liquid via Monte Carlo techniques: The effect of laser parameters and liquid medium on the electron cloud. Solid State Sci. 2022, 133, 107003.
- Povarnitsyn, M.E.; Itina, T.E.; Levashov, P.R.; Khishchenko, K.V. Mechanisms of nanoparticle formation by ultra-short laser ablation of metals in liquid environment. Phys. Chem. Chem. Phys. 2013, 15, 3108–3114.
- Ibrahimkutty, S.; Wagener, P.; Menzel, A.; Plech, A.; Barcikowski, S. Nanoparticle formation in a cavitation bubble after pulsed laser ablation in liquid studied with high time resolution small angle X-ray scattering. Appl. Phys. Lett. 2012, 101, 4750250.
- Dell’Aglio, M.; De Giacomo, A. Plasma charging effect on the nanoparticles releasing from the cavitation bubble to the solution during nanosecond Pulsed Laser Ablation in Liquid. Appl. Surf. Sci. 2020, 515, 146031.
- Taccogna, F.; Dell’Aglio, M.; Rutigliano, M.; Valenza, G.; De Giacomo, A. On the growth mechanism of nanoparticles in plasma during pulsed laser ablation in liquids. Plasma Sources Sci. Technol. 2017, 26, aa595b.
- Forsythe, R.C.; Cox, C.P.; Wilsey, M.K.; Müller, A.M. Pulsed Laser in Liquids Made Nanomaterials for Catalysis. Chem. Rev. 2021, 121, 7568–7637.
- Lu, L.; Zou, S.; Fang, B. The Critical Impacts of Ligands on Heterogeneous Nanocatalysis: A Review. ACS Catal. 2021, 11, 6020–6058.
- Qayyum, H.; Ahmed, W.; Hussain, S.; Khan, G.A.; Rehman, Z.U.; Ullah, S.; Rahman, T.U.; Dogar, A.H. Laser synthesis of surfactant-free silver nanoparticles for toxic dyes degradation and SERS applications. Opt. Laser Technol. 2020, 129, 106313.
- Mendivil Palma, M.I.; Krishnan, B.; Rodriguez, G.A.C.; Das Roy, T.K.; Avellaneda, D.A.; Shaji, S. Synthesis and Properties of Platinum Nanoparticles by Pulsed Laser Ablation in Liquid. J. Nanomater. 2016, 2016, 9651637.
- Charde, R.P.; Van Devener, B.; Nigra, M.M. Surfactant- and Ligand-Free Synthesis of Platinum Nanoparticles in Aqueous Solution for Catalytic Applications. Catalysts 2023, 13, 246.
- Kalus, M.-R.R.; Lanyumba, R.; Barcikowski, S.; Gökce, B. Discrimination of ablation, shielding, and interface layer effects on the steady-state formation of persistent bubbles under liquid flow conditions during laser synthesis of colloids. J. Flow Chem. 2021, 11, 773–792.
- Streubel, R.; Bendt, G.; Gökce, B. Pilot-scale synthesis of metal nanoparticles by high-speed pulsed laser ablation in liquids. Nanotechnology 2016, 27, 205602.
- Barcikowski, S.; Meńndez-Manjón, A.; Chichkov, B.; Brikas, M.; Račiukaitis, G. Generation of nanoparticle colloids by picosecond and femtosecond laser ablations in liquid flow. Appl. Phys. Lett. 2007, 91, 083113.
- Xiao, J.; Liu, P.; Wang, C.X.; Yang, G.W. External field-assisted laser ablation in liquid: An efficient strategy for nanocrystal synthesis and nanostructure assembly. Prog. Mater. Sci. 2017, 87, 140–220.
- Liang, Y.; Liu, P.; Yang, G. Fabrication of One-Dimensional chain of iron-based bimetallic alloying nanoparticles with unique magnetizations. Cryst. Growth Des. 2014, 14, 5847–5855.
- Ahmadinejad, E.; Mahdieh, M.H.; Hossein Mahdieh, M. Laser-assisted synthesis of Ag–Cu alloy nanoparticles with tunable surface plasmon resonance frequency in presence of external electric field. J. Laser Appl. 2021, 34, 012004.
- Sapkota, D.; Li, Y.; Musaev, O.R.; Wrobel, J.M.; Kruger, M.B. Effect of electric fields on tin nanoparticles prepared by laser ablation in water. J. Laser Appl. 2017, 29, 012002.
- Kanakkillam, S.S.; Krishnan, B.; Avellaneda, D.A.; Shaji, S. Surfactant free stable cobalt oxide nanocolloid in water by pulsed laser fragmentation and its thin films for visible light photocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124657.
- Nyabadza, A.; Vázquez, M.; Brabazon, D. Magnesium nanoparticle synthesis from powders via LASIS—Effects of liquid medium, laser pulse width and ageing on nanoparticle size, concentration, stability and electrical properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129651.
This entry is offline, you can click here to edit this entry!
