Flavonoids are small molecules, produced de novo by plants as secondary metabolites in response to diverse biotic and abiotic factors. These chemical compounds have a broad spectrum of established health-promoting effects. They are due to their antioxidative, anti-inflammatory, anti-mutagenic, and anti-carcinogenic properties coupled with their capacity to modulate key cellular enzyme functions. Flavonoids are widely distributed chemical compounds in the plant kingdom. Plants are therefore an inexhaustible source of flavonoids.
- flavonoids
- natural sources
1. Flavonoids—Classification
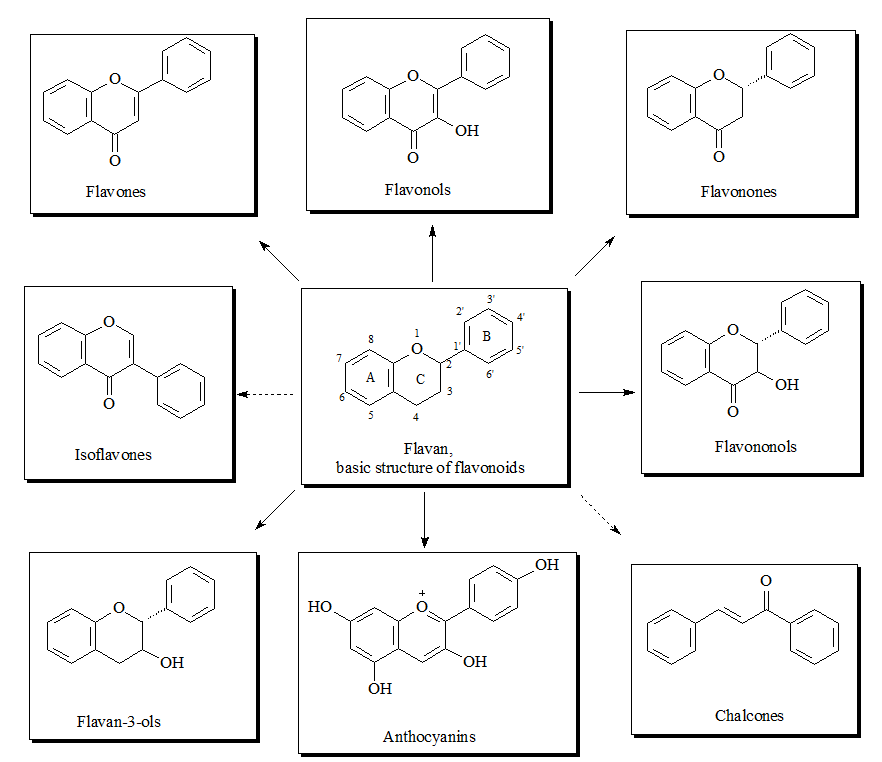
The flavonoid family includes more than 6000 low-molecular-weight phenolic compounds [1], derivatives of flavan. The main subgroups are flavones, flavonols, flavonones, flavononols, flavan-3-ols, anthocyanines, isoflavones, and chalcones (Figure 1).
Figure 1. Structures of flavonoid subgroups.
The flavan core is recognized in every single flavonoid structure. It consists of 15 carbon atoms which build two aromatic rings (commonly denoted as A and B) linked by a three-carbon chain. The connecting carbon chain is a part of a heterocyclic central ring (designed as C) and is available in the most flavonoids with one exception: in the structure of the chalcones the carbon chain between the A and B rings is linear [2]. For that reason the chalcones can be referred to as open-chain flavonoids.
Depending on the position of the linked B-ring to the benzopyrano (called also chromano) structure the flavones, flavonols, flavonones, flavononols, flavon-3-ols and anthocyanines, are 2-phenylbenzopyrans and the isoflavonoids are 3-phynylbenzopyrans, which makes this structures positional isomers. The flavonoids of the 2-phenylbenzopyran-subgroup differ in the degree of oxidation and saturation of the heterocyclic C-ring [3].
2. Natural Sources of Flavonoids
Flavonoids are small molecules, produced de novo by plants as secondary metabolites in response to diverse biotic and abiotic factors. They are widely distributed chemical compounds in the plant kingdom. Plants are therefore an inexhaustible source of flavonoids.
In 1936, the Hungarian scientist Szent-Gyorgyi (the discoverer of vitamin C) isolated a new substance from lemons. He called it “citrin” [3]. The structure of citrin was determined later: it is composed of the flavonoids hesperidin and eriodictyol [4]. Citrin was called also vitamin P. But, in 1950 was concluded that flavonoids did not meet the strict definition of a vitamin and the name vitamin P was taken off [5].
Their biochemical roles of the flavonoids in the plant are multifarious: from flower pigmentation to taking part in the growing processes, and the defense against diseases [6]. Each flavonoid group plays a unique biochemical role and has a particular distribution in plants [3].
The most popular edible plants rich in flavonoids, categorized by Tzanova et al. [7] in subgroups, are systematized in Tables 1.
Table 1. Food plants rich in flavonoids
|
Flavonoid subgroup |
Flavonoids |
Food |
References |
|
|
Flavonols and Flavan-3-ols |
Kaempferol; quercetin; myrecitin; (−)-epicatechin |
Black berries; wine |
|
|
|
Kaempferol; quercetin |
Tomato |
|
||
|
(+)-Catechin; (−)-epicatechin; epigallocatechin; chrysin; apigenin; quercetin; kaempferol |
Tea |
|
||
|
(+)-Catechin; (−)-epicatechin; quercetin |
Coffee; cocoa; apple |
|
||
|
Kaempferol; quercetin; myricetin; tamarixetin |
Onion; red wine; olive oil; berries; grapefruit; orange |
|
||
|
(+)-Catechin; (−)-epicatechin; quercetin; kaempferol |
Red berries; strawberries |
|
||
|
Quercetin |
Lemon; olive; aspargus |
|
||
|
Kaempferol |
Saffron spice |
[28] |
|
|
|
Kaempferol; quercetin |
Broccoli; brussel sprouts |
|
||
|
(+)-Catechin; (−)-epicatechin |
Apricot; nectarine; peach; plum; fig; banana; kiwi; hazelnut |
|
||
|
(+)-Catechin; (−)-epicatechin; quercetin; isorhamnetin; kaempferol |
Almond |
|
||
|
Flavones |
Luteolin |
Fruit skins; red wine; buckwheat; red pepper; tomato skin; lemon; watermelon; brussel sprouts; pumpkin |
||
|
Luteolin; apigenin; isorhoifolin |
Olive |
|
||
|
Flavonones |
Naringin; eriodictyol |
Almond |
[32] |
|
|
Naringin; maringenin; taxifolin; hesperitin; eriodictyol |
Citrus fruits; grapefruit; lemon; orange |
|
||
|
Anthocyanins |
Apigenidin; cyanidin |
Cherry; easberry; strawberry |
|
|
|
Cyanidin |
Olive |
|
||
|
Isoflavones |
Daidzin; genistein; glycitin; sissotrin; ononin |
Soya bean |
|
|
|
Biochanin A; formononetin |
Red clover |
[41] |
|
|
|
Genistin; daidzin; biochanin A |
Peanut |
[42] |
|
Fruits and vegetables are a rich diet source of flavonols and flavan-3-ols. Kaempferol, quercetin and myricetin are most studied flavonols, and catechin and epicatechin – the most studied flavan-3-ols. Because of their similar solubility in the polar alcoholic solvents, flavonols and flavan-3-ols were simultaneously extracted and detected, e.g. in black berries and wines [8][9][10]; in red berries [9][23][24]; in tee [13][15]; or coffee [9][16][17][18][19][20]. Onions, tomatoes, broccoli, apples and grapes are rich sources of flavonols [21][22].
Flavones are widely present in leaves, flowers and fruits as glucosides and their major sources are green leafy spices like parsley [43]. Luteolin is the most found flavone [22][26][31][32][33][34][35].
Flavanones are generally present in all citrus fruits [22][36][37]. Hesperitin, naringenin and eriodictyol are examples of this class of flavonoids. These compounds are responsible for the bitter taste of the juice and peel of citrus fruits. Almonds are rich also in naringin and eriodictyol [32].
Anthocyanins are pigments responsible for the coloring of flowers and fruits. Cyanidin is the most commonly studied anthocyanin. Various red and blue fruits are a rich diet sources of anthocyanins, which are concentrated in the fruit skins [21][22][25][26][35].
Isoflavones are found in legumes predominantly, e.g. soybean [38][39][40][41], but also in red clover [41] and in peanuts [42].
Major examples of chalcones include phloridzin, arbutin, phloretin, and chalconaringenin. Chalcones occur in significant amounts in tomatoes, pears, strawberries, bearberries, and certain wheat products [43].
Tzanova et al. [7] systemized the flavonoids detected in medicinal plants rich in their subgroups (Table 2).
Table 2. Medicinal plants rich in flavonoids
|
Flavonoid subgroup |
Flavonoids |
Medicinal Plant (Family) |
References |
|
|
Flavonols and Flavan-ols |
(+)-Catechin |
Brysonima crassa (Compositae) |
[44] |
|
|
Isorhamnetin |
Calendula officinalis (Compositae) |
[45] |
|
|
|
Kaempferol |
Acalypha indica (Euphorbiaceae); Clitoria ternatea (Fabaceae); Pteris vittata L (Pteridaceae) |
|
||
|
Quercetin |
Betula pendula (Betulaceae) Bauhinia monandra (Fabaceae); Pteris vittata L (Pteridaceae) Cannabis sativa (Compositae); Azadirachta indica (Meliaceae); Angelica L. (Apiaceae); |
|
||
|
Hyperoside |
Tilia cordata (Tiliaceae) |
[45] |
|
|
|
Isoquercetin |
Mimosa pudica (Mimosoideae) |
[52] |
|
|
|
Pongaflavonol |
Pongamia pinnata (Fabaceae) |
[53] |
|
|
|
2-(3, 4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one |
Chenopodium album L. (Chenopodiaceae) |
[54] |
|
|
|
2-(3,4-dihydroxy-5-methoxy-phenyl)-3,5-dihydroxy-6,7-dimethoxychromen-4-one |
Euphorbia neriifolia (Euphorbiaceae) |
[55] |
|
|
|
Flavones |
Pectolinarigenin |
Clerodendrum phlomidis (Verbenaceae) |
[49] |
|
|
Luteolin |
Aloe vera (Asphodelaceae); Momordica charantia (Curcurbitaceae); Bacopa moneirra (Scrophulariaceae); Angelica L. (Apiaceae); Mentha longifolia (Lamiaceae) |
|
||
|
Hispidulin; apigenin; cirsimaritin |
Rosmarinus officinalis L. (Lamiaceae) |
|
||
|
Luteolin; hispidulin; apigenin; cirsimaritin |
Salvia officinalis L. (Lamiaceae) |
[58] |
|
|
|
Luteolin; hispidulin |
Thymus L. (Lamiaceae) |
|
||
|
Apigenin; hispidulin |
Verbena officinalis L. (Verbenaceae) |
[60] |
|
|
|
5-hydroxy-7,8-dimethoxyflavone |
Andrographis paniculata (Acanthaceae) |
[45] |
|
|
|
3,4-methlenedioxyflavone |
Limnophila indica (Scrophulariaceae) |
[52] |
|
|
|
Chrysin |
Oroxylum indicum (Bignoniaceaea) |
[52] |
|
|
|
Vitexin |
Passiflora incarnate (Passifloraceae) |
[45] |
|
|
|
Flavonones |
Narginin |
Rosmarinus officinalis L. (Lamiaceae) |
|
|
|
Hesperidin |
Citrus medica (Rutaceae) |
[46] |
|
|
|
Liquiritin |
Glyccheriza glabra (Leguminosae) |
[45] |
|
|
|
Kurarinol; kurarinone |
Sophora flavescens Ait. (Fabaceae) |
[61] |
|
|
|
Flavononols |
Kushenol I; kushenol N |
Sophora flavescens Ait. (Fabaceae) |
[61] |
|
|
Isoflavones |
Genistein |
Calopogonium muconoides (Fabaceae) Butea monospermea (Fabaceae); Andira macrothyrsa (Fabaceae); |
[41] [51] |
|
|
Biochanin A |
Cratylia argentea (Fabaceae); A. macrothyrsa (Melastomataceae) |
[41] |
|
Among the medicinal plants the Fabaceae family are mostly investigated: flavonoles are found in Bauhinia monandra [45], Clitoria ternatea [47], and Pongamia pinnata [53]; flavonones and flavononols – in Sophora flavescens Ait [51]. Best represented are of course the isoflavones – in Calopogonium muconoides, Butea monospermea, Andira macrothyrsa, and Cratylia argentea [41][51].
Lamiaceae family is also good presented: plants from this family are rich source of flavones and flavonones, for example Rosmarinus officinalis L. [58][59]. From other members of this family are isolated flavones: Salvia officinalis L. [58], Thymus L. [58][60], and Mentha longifolia [57]. Flavonoles and flavones are the wide distributed in the medicinal plants flavonoids, just like in the food plants.
This entry is adapted from the peer-reviewed paper 10.3390/pr8101222
References
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504.
- Beecher, G.R. J. Nutr. 2003, 133, 3248S
- Alzand, K.I.; Mohamed, M.A. Flavonoids: Chemistry, Biochemistry and Antioxidant activity. J. Pharm. Res. 2012, 5, 4013–4020.
- Harborne, J.B. The Flavonoids: Advances in Research Since 1986, Chapman and Hall, London, 1994.
- Strack, D., Wray, V. The Anthocyanins, In: The Flavonoids: Advances in Research since 1986, Harborne, J.B., Ed., Chapman and Hall, London, 1994.
- Havsteen, B. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202.
- Tzanova, M., Atanasov, V., Yaneva, Z., Ivanova, D., Dinev, T. Selectivity of Current Extraction Techniques for Flavonoids from Plant Materials. Processes 2020, 8(10), 1222
- Ollanketo, M.; Riekko,la, M.L. Column-switching technique for selective determination of flavonoids in Finnish berry wines by high-performance liquid chromatography with diode array detection. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 1339–1351.
- Arts, I.C.W.; van de Putte, B.; Hollman, P.C.H. Catechin Contents of Foods Commonly Consumed in The Netherlands. 1. Fruits, Vegetables, Staple Foods, and Processed Foods. J. Agric. Food Chem. 2000, 48, 1746–1751.
- de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 2000, 48, 5331–5337.
- Crozier, A.; Lean, M.E.J.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoids content of commercial tomatoes, onions, lettuce and celery. J. Agric. Food Chem. 1997, 45, 590–595.
- Raffo, A.; la Malfa, G.; Fogliano, V.; Maiani, G.; Quaglia, G. Seasonal variations in antioxidant components of cherry tomatoes (Lycopersicon esculentum cv. Naomi F1). J. Food Compost. Anal. 2006, 19, 11–19.
- Hara, Y.; Luo, S.J.; Wickremasinghe, R.L.; Yamanishi, T. Special issue on tea. Food Rev. Int. 1995, 11, 371–542.
- Xu, J.Z.; Leung, L.K.; Huang, Y.; Chen, Z.Y. Epimerisation of tea polyphenols in tea drinks. J. Sci. Food Agric. 2003, 83, 1617–1621.
- Price, K.R.; Rhodes, M.J.C.; Barnes, K.A. Flavonol glycoside content and composition of tea infusions made from commercially available teas and tea products. J. Agric. Food Chem. 1998, 46, 2517–2522.
- Natsume, M.; Osakabe, N.; Yamagishi, M.; Takizawa, T.; Nakamura, T.; Miyatake, H.; Hatano, T.; Yoshida, T. Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC. Biosci. Biotechnol. Biochem. 2000, 64, 2581–2587.
- Gu, L.; House, S.E.; Wu, X.; Ou, B.; Prior, R.L. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J. Agric. Food Chem. 2006, 54, 4057–4061.
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marin, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935.
- Lee, K.W.; Kim, Y.J.; Kim, D.O.; Lee, H.J.; Lee, C.Y. Major phenolics in apple and their contribution to the total antioxidant capacity. J. Agric. Food Chem. 2003, 51, 6516–6520.
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538.
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.; Crozier, A. Occurrence of flavonols in tomatoes and tomato-based products. J. Agric. Food Chem. 2000, 48, 2663–2669.
- Lugasi, A.; Hovari, J. Flavonoid aglycons in foods of plant origin. II. Fresh and dried fruits. Acta Aliment. 2002, 31, 63–71.
- Mullen, W.; Stewart, A.J.; Lean, M.E.J.; Gardner, P.; Duthie, G.G.; Crozier, A. Effect of freezing and storage on the phenolics, ellagitannins, flavonoids, and antioxidant capacity of red raspberries. J. Agric. Food Chem. 2002, 50, 5197–5201.
- Wada, L.; Boxin, O. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500.
- Maatta-Riihinen, K.R.; Kamal-Eldin, A.; Torronen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187.
- Romani, A.; Mulinacci, N.; Pinelli, P.; Vincieri, F.F.; Cimato, A. Polyphenolic content in five Tuscany cultivars of Olea europaea L. J. Agric. Food Chem. 1999, 47, 964–967.
- Maeda, T.; Kakuta, H.; Sonoda, T.; Motoki, S.; Ueno, R.; Suzuki, T.; Oosawa, K. Antioxidation capacities of extracts from green, purple, and white asparagus spears related to polyphenol concentration. HortScience 2005, 40, 1221–1224.
- Carmona, M.; Sanchez, A.M.; Ferreres, F.; Zalacain, A.; Tomas-Barberan, F.; Alonso, G.L. Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: Comparative study of samples from different geographical origins. Food Chem. 2007, 100, 445–450.
- Chang, S.; Tan, C.; Frankel, E.N.; Barrett, D.M. Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol oxidase activity in selected clingstone peach cultivars. J. Agric. Food Chem. 2000, 48, 147–151.
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977.
- Milbury, P.E.; Chen, C.Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033.
- Kreft, S.; Knapp, M.; Kreft, I. Extraction of rutin from buckwheat (Fagopyrum esculentum Moench) seeds and determination by capillary electrophoresis. J. Agric. Food Chem. 1999, 47, 4649–4652.
- Hertog, M.G.L.; Hollman, P.C.H.; Katan, M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992, 40, 2379–2383.
- Vlahov, G. Flavonoids in three olive (Olea europaea) fruit varieties during maturation. J. Sci. Food Agric. 1992, 58, 157–159.
- Miyake, Y.; Shimoi, K.; Kumazawa, S.; Yamamoto, K.; Kinae, N.; Osawa, T. Identification and antioxidant activity of flavonoid metabolites in plasma and urine of eriocitrin-treatedrats. J. Agric. Food Chem. 2000, 48, 3217–3224.
- Rousseff, R.L.; Martin, S.F.; Youtsey, C.O. Quantitative survey of narirutin, naringin, hesperidin, and neohesperidin in citrus. J. Agric. Food Chem. 1987, 35, 1027–1030.
- Justesen, U.; Knuthsen, P.; Leth, T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A 1999, 799, 101–110.
- Reinli, K.; Block, G. Phytoestrogen content of foods: A compendium of literature values. Nutr. Cancer 1996, 26, 123–148.
- Wiseman, H.; Casey, K.; Clarke, D.B.; Barnes, K.A.; Bowey, E. Isoflavone aglycon and glucoconjugate content of high- and low-soy UK foods used in nutritional studies. J. Agric. Food Chem. 2002, 50, 1401–1410.
- Chen, X.J.; Zhao, J.; Meng, Q.; Li, S.P.; Wang, Y.T. Simultaneous determination of five flavonoids in licorice using pressurized liquid extraction and capillary electrochromatography coupled with peak suppression diode array detection. J. Chrom. A 2009, 1216, 7329–7335.
- Leuner, O.; Havlik, J.; Hummelova, J.; Prokudina, E.; Novy, P.; Kokoska, L. Distribution of isoflavones and coumestrol in neglected tropical and subtropical legumes. J. Sci. Food Agric. 2013, 93, 575–579.
- Chukwumah, Y.C.; Walker, L.T.; Verghese, M.; Bokanga, M.; Ogutu, S.; Alphonse, K. Comparison of extraction methods for the quantification of selected phytochemicals in peanuts (Arachis hypogaea). J. Agric. Food Chem. 2007, 55, 285–290.
- Panche, A.N., Diwan, A.D., Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47, 1-15.
- Abdullah, S.; Shaari, A.R.; Azimi, A. Effect of Drying Methods on Metabolites Composition of Misai Kucing (Orthosiphon stamineus) Leaves. APCBEE Procedia 2012, 2, 178.
- Gupta, K.K.; Taneja, S.C.; Dhar, K.L.; Atal, C.K. Flavonoids of Andrographis paniculata. Phytochemistry 1983, 22, 314–315.
- L’azaro, M.L. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. 2009, 9, 31–59.
- Sankaranarayanan, S.; Bama, P.; Ramachandran, J.; Kalaichelvan, P.T.; Deccaraman, M.; Vijayalakshimi, M.; Dhamotharan, R.; Dananjeyan, B.; Bama, S.S. Ethnobotanical study of medicinal plants used by traditional users in Villupuram district of Tamil Nadu, India. J. Med. Plant Res. 2010, 4, 1089–1101.
- Lin, L.J.; Huang, X.B.; Lv, Z.C. Isolation and identification of flavonoids components from Pteris vittata L. SpringerPlus 2016, 5, 1649.
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479.
- Lee, J.H.; Lee, J.Y.; Kim, K.N.; Kim, H.S. Quantitative analysis of two major flavonoid aglycones in acid hydrolyzed samples of Angelica keiskei by HPLC. Food Sci. Biotechnol. 2003, 12, 415–418.
- Murlidhar, A.; Babu, K.S.; Sankar, T.R.; Redenna, P.; Reddy, G.V.; Latha, J. Antiinflammatory activity of flavonoid fraction isolated from stem bark of Butea monosperma (Lam): A mechanism based study. Int. J. Phytopharm. 2010, 1, 124–132.
- Sannomiya, M.; Fonseca, V.B.; Silva, M.A.D.; Rocha, L.R.M.; Dos Santos, L.C.; Hiruma-Lima, C.A.; Souza Brito, A.R.M.; Vilegas, W. Flavonoids and antiulcerogenic activity from Byrsonima crassa leaves extracts. J. Ethnopharm. 2005, 97, 1–6.
- Agarwal, M.; Kamal, R. Studies on flavonoid production using in-vitro cultures of Momordica charantia. Indian J. Biotechnol. 2007, 6, 277–279.
- Arora, S.; Itankar, P. Extraction, isolation and identification of flavonoid from Chenopodium album aerial parts. J. Tradit. Complement. Med. 2018, 8, 476–482.
- Sharma, V.; Janmeda, P. Extraction, isolation and identification of flavonoid from Euphorbia neriifolia leaves. Arab. J. Chem. 2017, 10, 509–514.
- Ghoulami, S.; Idrissi, A.I.; Fkih-Tetouani, S. Phytochemical study of Mentha longifolia of Morocco. Fitoterapia 2001, 72, 596–598.
- Kogawa, K.; Kazuma, K.; Kato, N.; Noda, N.; Suzuki, M. Biosynthesis of malonylated flavonoid glycosides on basis of malonyl transferase activity in the petals of Clitoria ternatea. J. Plant Physiol. 2007, 164, 886–894.
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170.
- Luis, J.C.; Johnson, C.B. Seasonal variations of rosmarinic and carnosic acids in rosemary extracts. Analysis of their in vitro antiradical activity. Span. J. Agric. Res. 2005, 3, 106–112.
- Mueller, A.; Ganzera, M.; Stuppner, H. Analysis of the aerial parts of Verbena officinalis L. by micellar electrokinetic capillary chromatography. Chromatographia 2004, 60, 193–197.
- Zhang, Q.; Yu, J.; Wang, Y.; Su, W. Selective Extraction of Flavonoids from Sophora flavescens Ait by Mechanochemistry. Molecules 2016, 21, 989.