Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Reproductive Biology
Epidemiological studies have suggested that arsenic is one of the most hazardous reproductive toxicants present in the environment, which is significantly accumulated in the reproductive tissues like the testes, epididymis, seminal vesicle, and prostate gland. Arsenic exposure exerted cellular and molecular perturbations such as oxidative stress, inflammation, induction of autophagy, and apoptosis, which obstructed male gonadal development and led to reproductive dysfunction in humans and animals.
- arsenic
- metalloid
- phytonutrients
- reproductive toxicity
- molecular mechanisms
1. Introduction
Arsenic (As) is a naturally occurring toxic metalloid with odourless, colourless, and tasteless properties. The most commonly available forms of arsenic include inorganic As, organic As, and arsine gas [1]. Arsenic is ranked as the 20th most abundant element in the terrestrial, 14th in the marine, and 12th in the human ecosystem. It possesses significant health concerns due to its ubiquitous existence, discharged into the environment from volcanic and industrial activities [2,3]. Charles Dickens used arsenic as a tonic, called Fowler’s solution (potassium arsenate in water). It was also employed to treat leukaemia, psoriasis, and chronic bronchial asthma [4,5]. As is a well-known poison whose use is restricted to the production of pesticides, herbicides, cotton desiccants, and exfoliants in agriculture, applied as doping material in the semiconductor industry, bronzing, pyrotechnics, as well as the manufacturing of special kinds of glasses and preservation of wood [6,7]. As is found naturally in both trivalent and pentavalent forms, but the pentavalent form is much more common and is the best form for eliminating arsenic from biological systems [8,9]. The commonly available pentavalent forms of arsenic are arsenic pentoxide, arsenic acid, sodium arsenate, lead arsenate, and calcium arsenate [10,11]. More than 200 million people are adversely affected by toxic responses to As, which acknowledges research and mitigation methods to control human arsenic exposure [12]. Since it is ubiquitous, humans are exposed via groundwater, food, and industrial and anthropogenic sources [13]. The largest source of As exposure for people is drinking water; the World Health Organization (WHO) and Environmental Protection Agency (EPA) recommend a maximum concentration of 10 µg/L for As in drinking water [14]. As exposure through the air is negligible, effects are observed when the air comprises a mixture of arsenite and arsenate [14]. As mobilisation in drinking water results from microbial reactions such as oxidizing arsenite or reducing arsenate [15]. Furthermore, arsine gas, gallium arsenide, glass manufacturing facilities, and coal-fired power plants are some of the occupational sources of arsenic [16]. Furthermore, elevated levels of arsenic were found in commonly consumed foods such as grains, vegetables, and rice, as well as significant concentrations in meat products such as beef, poultry, and shellfish [17]. Several countries around the world are highly exposed and vulnerable to arsenic toxicity, including Cambodia, China, India, Mexico, Pakistan, the United States, Vietnam, and East Croatia [18,19,20]. Chronic exposure in Bangladesh and Taiwan, as well as low-level exposure in the United States, has resulted in the onset of type II diabetes [21,22]. Pregnant women in the Chilean region who were exposed to As in their drinking water for an extended period of time experienced a decrease in baby birth weight [23]. Cancer, genetic changes, and dermatological diseases have been reported in Argentina when As concentrations reach 100 µg/L [24]. In Taiwan, As contamination in drinking water increases the risk of lung, kidney, and bladder cancer [25]. As levels in the aquifer have the greatest impact on a few provinces in India (Uttar Pradesh, Bihar, and West Bengal) and Bangladesh [26,27,28,29].
As is designated by the International Agency for Research on Cancer (IARC) as a group-I carcinogen that also raises the risk of bladder, lung, kidney, and liver cancer [25,30,31,32].
As induces carcinogenesis by epigenetic modifications in miRNA expression [33,34], DNA methylation, and histone modifications [35]. Low doses of As in the form of orpiment (AS2S3), realgar (AS4S4), and especially arsenolite (contains arsenic trioxide, AS2O3) have been used as a therapeutic agent in Iranian traditional medicine since Avicenna (1023 A.D.) [36,37]. Interestingly, As was therapeutically used to treat chronic myelogenous leukaemia (CML) until radiation and chemotherapy took over [9]. As has also demonstrated significant success in the treatment of newly diagnosed and relapsed individuals with acute promyelocytic leukaemia (APL) [38]. Acute exposure to As causes nausea, vomiting, abdominal pain, and severe diarrhoea, whereas prolonged exposure causes damage to multiple organs [11,39]. Interestingly, Calabrese and Baldwin (2009) found hormetic dose–response relationships with inorganic compounds including arsenic showed that mechanisms associated with arsenic may decipher the new dimensions. Recently, Ommati et al., found that in mature F1 male mice, the spermatogenic index was higher in low-dose (0.2 ppm) animals, demonstrating another possible hormesis effect, but a dose-dependent decrease in the spermatogenic index was found at higher doses (2 and 20 ppm) of As2O3. It is interesting to note that Calabrese and Baldwin (2009) discovered hormetic dose–response relationships with inorganic compounds, one of which was arsenic. These relationships revealed that mechanisms associated with arsenic may be able to decipher new dimensions [40]. Ommati et al. (2019) recently found that in mature F1 male mice, the spermatogenic index was higher in low-dose (0.2 ppm) animals, demonstrating another possible hormesis effect, whereas a dose-dependent decrease in the spermatogenic index was found at higher doses (2 and 20 ppm) of As2O3 [41]. In addition, a hormesis effect was seen in female mice, as indicated by a decrement in thiobarbituric acid reactive substances (TBARS) content and an increase in ovarian mammalian target of rapamycin (mTOR) gene expression level, both of which occurred at lower doses of As2O3 [42]. They also observed the hormesis effect in the hypothalamic–pituitary–gonadal (HPG axis) of pubertal male offspring when exposed to low levels of As2O3 (0.2 ppm) [37].
2. Exposure to Arsenic in the Environment
Arsenic exposure in humans is constantly rising in drinking water, food, and industrial sources. Higher arsenic levels were reported in drinking water in most countries and were higher than the specified limits of WHO and EPA. Cultivation of rice with As-contaminated soil and the use of As-contaminated water to cook rice are the major contributors to As exposure in cooked rice [71]. Finfish, shellfish, and seaweed are the most common sources of As exposure in humans through seafood [72]. As depicted in Table 1, urine, hair, and drinking water samples collected from children and adults from As-contaminated regions in Argentina, Uruguay, India, Pakistan, and Spain showed higher levels of arsenic. Accumulation levels of arsenic in various foods like rice, meat, cereals, and vegetables in different regions of the world were presented in Table 2. Overall, there is a significant risk of cancer in children and adults due to the build-up of arsenic in the diet.
Table 1. Compilation of research studies showing the effect of arsenic exposures among diverse demographics in various parts of the world.
| S. No | Countries | Population (Subjects) | Sample Size | Sample | Detected Levels of Arsenic | Reference |
|---|---|---|---|---|---|---|
| 1 | Argentina | Children (3–15 years) | 101 | Hair, Urine | 110–1311 μg/kg | [73] |
| 2 | Montevideo, Uruguay | Children (5–8 years) | 328 | Drinking water | 9.9 μg/L | [74] |
| 0.45 μg/L | ||||||
| 3 | Shaanxi province, China | Adults | 96 | Drinking water | 4.52 µg/L | [75] |
| Indoor air | 0.03 mg/m3 | |||||
| Soil | 14930 mg/kg | |||||
| 4 | Inner Mongolia | Adults | 96 | Drinking water | 144.71 µg/L | |
| Soil | 10190 mg/kg | |||||
| 5 | Villages of Pakistan | Children (≤16 years) | 223 | Ground water | 15. 63 µg/kg/day (arsenate) 0.09 µg/kg/day (arsenite) | [76] |
| Adult | 15.07 µg/kg/day (arsenate) 0.26 µg/kg/day (arsenite) | |||||
| 6 | Indae metal mine area | Residents (mean age of 66.8 years) | 50 | Urine | Arsenite (1.45 µg/L), arsenate (0.74 µg/L), MMA (2.43 µg/L), DMA (27.63 µg/L), and arsenobetaine (88.62 µg/L) | [77] |
| 7 | Spain | Children (4–5 years) | 400 | Urine | 2.74–7.54 µg/L | [78] |
Table 2. Evidence from research findings indicates the presence of arsenic in grains, vegetables, dairy products, and meat which increases the risk of cancer.
| S. No | Country | Sample (s) | Arsenic Levels | Reference |
|---|---|---|---|---|
| 1 | Kolkata, India | Rice, grain, and vegetable | 76 µg/kg and 41.4 µg/kg | [79] |
| 2 | UK | Rice | 130 μg/kg | [80] |
| 3 | Kunming, China | Rice | 3520 µg/kg | [81] |
| 4 | Japan | Rice, hijiki | 19 µg/kg and 59 µg/kg | [82] |
| 5 | Pakistan | Raw rice | 92.5 ± 41.88 μg/kg | [83] |
| Cooked rice | 79.21 ± 76.42 μg/kg | |||
| Wheat | 116.38 ± 51.38 μg/kg | |||
| 6 | West Bengal, India | Boro and Aman rice | 194 μg/kg and 156 μg/kg | [79] |
| Arum and radish | 780 and 674 μg/kg | |||
| Urine | 154–276 µg/L | |||
| 7 | Ngari area, Western Tibet | Barley | 180 ± 210 µg/kg | [84] |
| Vegetable | 400 ± 570 µg/kg, | |||
| Meat | 210 ± 160 µg/kg | |||
| Dairy products | 180 ± 80 µg/kg | |||
| Daily intake | 3600 µg/kg | |||
| 8 | Kurdistan province, Iran | Sheep meat | 230 ± 140 µg/kg | [85] |
| Beef meat | 200 ± 90 µg/kg | |||
| Turkey meat | 880 ± 210 µg/kg | |||
| Ostrich meat | 850 ± 130 µg/kg |
3. Metabolism of Arsenic
In many organisms, along with humans, the inorganic form of As gets rapidly reduced from arsenate (pentavalent) to arsenite (trivalent) in the blood, facilitated by glutathione (GSH) [86]. The latter is 2–10 folds more toxic and rapidly taken up by the cells than the former [10]. Once absorbed, arsenite binds to globin of Hb- and -SH-containing proteins such as Glutathione and Cysteine and is distributed to the skin, hair, and mucosa owing to thiol-cystine amino acids. Skin, hair, epithelium of gastrointestinal tract (GIT), and epididymis had the highest As retention [87,88,89,90]. Aquaglyceroporins (AQPs) are involved in transporting As into cells [91], whereas efflux is carried out by major facilitator superfamily (MFS) transporters and ATP-binding cassette (ABC) transporters [92]. As shown in Figure 1, initially, biotransformation of arsenic involves oxidative methylation of arsenite to arsenate, mediated by the enzyme arsenite methyltransferase (As3MT), a primary methyl donor, followed by oxidation, arsenite to a pentavalent metabolite of arsenate, monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA) [93,94]. MMA and DMA were taken up less effectively by organs and tissue than arsenite and rapidly excreted into urine [95]. In contrast, evidence suggests that metabolites of As tend to induce chromosomal mutations [96] and show genotoxicity [97]. Methylated forms of As were also found in human urine, natural water, and bird eggshells [98].
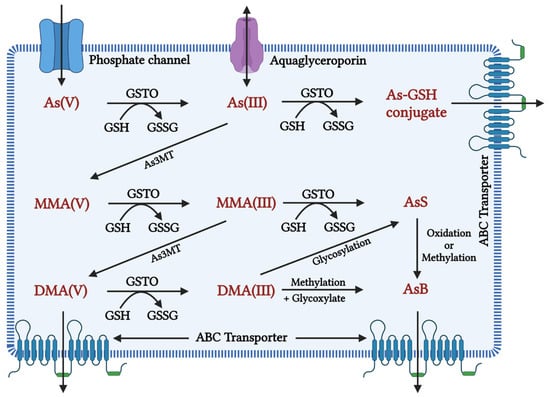
Figure 1. Inorganic arsenic conversion pathways into mono-, di-, and trimethylated products when the toxic form of As(III) is taken up by a cell, it is methylated to MMA (monomethylarsonic acid) and DMA (dimethylarsinic acid) with the help of the enzyme arsenic methyl transferase (AsMT) and can then be eliminated from the body through urine. However, in a pathological state, SAM depletion causes As(III) aggregation, affecting cellular homeostasis in a variety of ways, including oxidative balance, inflammation, and genetic and epigenetic processes. This disruption in homeostasis causes cellular damage and, eventually, cell death. As(V): inorganic pentavalent arsenic; As(III): inorganic trivalent arsenic; As3MT: arsenite methyltransferase, MMA(V): methyl arsonate; MMA(III): monomethylarsonous acid; DMA(V): dimethyl arsenate; DMA(III): dimethylarsinous acid; GSH: glutathione; GSTO: Glutathione S-transferase omega, GSSG: Glutathione disulfide As-GSH: Arsenic glutathione; AsS: Arsenic sulphide; AsB: Arsenobetaine; ABC transporter: ATP-binding cassette transporters; AS3MT: arsenite methyltransferase.
4. Arsenic-Induced Oxidative Stress and DNA Damage
At a biochemical level, inorganic arsenic in the pentavalent state replaces the phosphate in several reactions, the trivalent form of inorganic and organic (methylated) arsenic reacts with critical thiols and inhibits their activity [93]. As induces the formation of reactive oxygen species (ROS), enhances free radicals, and diminishes the activity of thiol group-rich antioxidants such as glutathione (GSH) [99]. Production of ROS will take place during the generation of intermediate arsine species [100]. Methylated arsenicals cause the release of iron from ferritin and initiate the production of hydroxy radicals and progress ferroptosis [101]. Arsenic shows mitochondrial toxicity by inhibiting succinic dehydrogenase activity and uncoupling oxidative phosphorylation with the production of O2, which gives rise to other forms of ROS [102]. Oxidative stress leading to the activation of ERK/AKT/NF-kβ pathway is one of the primary molecular mechanisms involved in arsenic-induced male reproductive toxicity [10]. Mitogen-activated protein kinases (MAPKs) are proteins that regulate the signalling of cells in response to environmental stimuli. The ERK signalling pathway is involved in a variety of male reproductive functions, such as spermatogenesis and Sertoli cell function. Activation of ERK1/2 inhibits the function of Sertoli cells and increases apoptosis in the testes. Protein kinase B (AKT) regulates oxidative stress in conjunction with the immune system by regulating cell growth, survival, proliferation, and inflammation. MAPK and AKT both directly phosphorylate nuclear factor kappa B (NF-B), which increases NF-ĸB binding to target DNAs and the expression/activity of NF-ĸB-controlled genes. Spermatogenesis and the function of Sertoli cells in the testes are governed by the transcription factor NF-ĸB. Activation of NF-ĸB inhibits spermatogenesis in both humans and mice. Arsenic activated the ERK/AKT/NF-B signalling pathways in numerous cell types. The expression of ERK1/2, IKK, PI3K, AKT, and NF-B, as well as the phosphorylation of ERK/AKT, increased in rats exposed to sodium arsenite (1, 5, or 25 mg/L) for 6 months. This causes reproductive toxicity via ERK/AKT/NF-kB signalling [68,69,70,103,104,105,106,107]. Figure 2 depicts As-induced oxidative stress mechanisms.
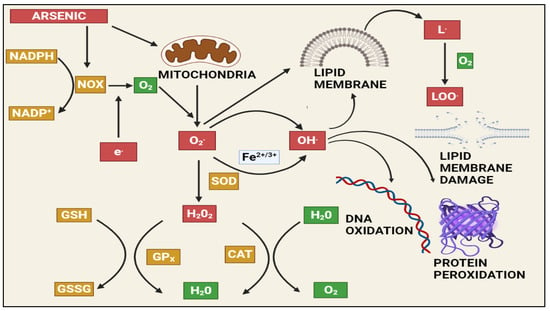
Figure 2. The effects of arsenic-induced mitochondrial reactive oxygen species generation. Arsenic generates a significant amount of ROS, primarily through complexes I and II of the electron transport chain (ETC). The ETC produces superoxide radicals, which react with other radicals in the cell to form stable and long-lived reactive species that damage macromolecules and induce apoptosis via various pathways. NAPDH: Nicotinamide adenine dinucleotide phosphate (NADP+), NADPH oxidase (NOX), GSH: Gluthathione, GSSG: Glutathione disulfide, GPx: Glutathione peroxidase, SOD: Superoxide dismutase, CAT: Catalase, H2O2: Hydrogen peroxide, LOO: lipid peroxy radical.
5. Effects of Arsenic on the Male Reproductive System in Animals
Epidemiological studies have suggested that arsenic is one of the most hazardous reproductive toxicants present in the environment, which is significantly accumulated in the reproductive tissues like the testes, epididymis, seminal vesicle, and prostate gland [108,109]. The inhibition of testosterone biosynthesis by arsenic is depicted in Figure 3. Arsenic exposure exerted cellular and molecular perturbations such as oxidative stress, inflammation, induction of autophagy, and apoptosis, which obstructed male gonadal development and led to reproductive dysfunction in humans and animals [110,111]. Arsenic has been shown to damage the histology of various tissues, such as the liver, brain, and kidney [112,113]. Notably, arsenic exposure during development significantly altered tight junctions’ proteins, leading to an increase in blood–brain barrier permeability [114]. As a result, the age-dependent inhibition of the PI3K/Akt/mTOR signalling pathway may contribute to the induction of autophagy and the facilitation of arsenic transfer through the cerebellum’s cerebral cortex and hippocampus leaky blood–brain barrier [115]. Recent studies from the Ommati research group also shed some light on hypothalamic–pituitary–gonadal (HPG) axis disruption and subsequent toxicity in mice and its continued impact on offspring as well (refer to Figure 3) [37,41,43,116,117]. Similarly, the blood–testis barrier (BTB) is one of the most impermeable blood–tissue barriers in mammals. It divides the seminiferous epithelium into basal (intraluminal) and apical (intraluminal) compartments. Meiosis I and II, spermiogenesis, and spermiation all occur in a specialised microenvironment behind the BTB in the apical compartment, whereas spermatogonial renewal and differentiation, as well as cell cycle progression up to the preleptotene spermatocyte stage, occurs outside the BTB in the basal compartment of the epithelium. However, the BTB is not a static ultrastructure. Instead, it undergoes extensive remodelling during stage VIII of the seminiferous epithelial cycle of spermatogenesis to allow preleptotene spermatocytes to pass through the BTB. However, the BTB’s immunological barrier cannot be compromised, even temporarily, during the epithelial cycle in order to prevent the production of antibodies against meiotic and postmeiotic germ cells. Adhesion protein complexes (e.g., occludin-ZO-1, N-cadherin—catenin, claudin-5-ZO-1), steroids (e.g., testosterone, estradiol-17), nonreceptor protein kinases (e.g., focal adhesion kinase, c-Src, c-Yes), polarity proteins induce testicular damage via their initial actions at the BTB, resulting in germ-cell loss, reduced sperm count, and male infertility or subfertility. Metallothioneins [cysteine-rich low molecular weight metal-binding proteins localised to the membrane of the Golgi apparatus that protect cells from cytotoxicity of essential heavy metals (such as zinc, selenium, and copper) and non-essential heavy metals (such as arsenic, mercury, silver, and cadmium) by binding to these metals via the thiol groups] are largely responsible for heavy metal accumulation in the body. As a result, significant and harmful amounts of heavy metal accumulation can accumulate in a person over time, exceeding the capacity of metallothioneins in the process [118,119,120,121,122,123].
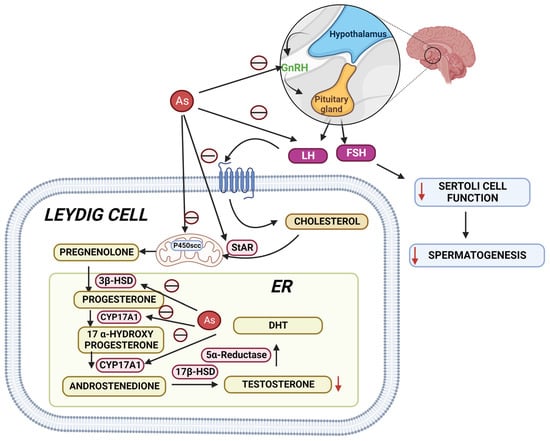
Figure 3. Arsenic’s mechanisms show that it alters the hypothalamic–pituitary–gonadal (HPG) axis, resulting in a decrease in testosterone biosynthesis, Sertoli cell activity, and spermatogenesis. StAR: steroidogenic acute regulatory protein; 3HSD: 3-beta (β)-hydroxysteroid dehydrogenase; CYP17A1: Cytochrome P450 17A1; 17HSD: 17-beta (β)-hydroxysteroid dehydrogenase; DHT: Dihydrotestosterone.
Testicular histopathological investigations showed that sodium arsenite decreased seminiferous tubule diameter in Wistar rats [16] and outbred Institute of Cancer Research (ICR) mice led to a significant decrease in the lumen in the arsenic-treated group compared to the control group [124]. Vacuolisation, acidophilic cells, and epithelial degeneration were associated with increased inflammatory cytokines in male rats exposed to sodium arsenite through drinking water [125]. Moreover, sodium arsenite exhibits severe damage to the testicular structure and elevated cleaved caspase 3 (CC3) which is an apoptotic marker in the cluster differentiation 1 (CD1) mouse testes cell line [126]. Increased expression of CC3 also indicates increased apoptosis. Together, sodium arsenite and arsenate at a concentration of 0.01 and 10 mg/L in drinking water showed a decrease in catalase activity and vacuolisation of seminiferous tubules in rats [127]. Arsenic exposure inhibited the spermatogenesis process and decreased the mobility and viability of sperm [128]. An increase in apoptotic spermatozoa has been observed with arsenic exposure, the central mechanism involved in arsenic-induced decreased sperm count [129]. Sodium arsenite exposure at a concentration of 10 mg/L through drinking water for eight weeks exhibited a decrease in sperm counts and enhanced sperm head abnormalities, which led to an increase in the infertility risk and pre-implantation loss in Wistar rats [130]. Moreover, in mice, arsenic trioxide at doses of 0.3 and 3 mg/kg s.c. for 35 days reduced the number of spermatozoa, increased epithelial aberration and exfoliation of germ cells in the tubule lumen, and altered the nucleus/cytoplasm ratio of Leydig cells [131]. In mouse testes, arsenic trioxide at concentrations of 0.2, 2, and 20 ppm in drinking water for six months impaired sperm motility and affected the ultra-structure of the acrosome structure and sperm tail by downregulating the protein expression of DPY19L2, AKAP3, CFAP44, and SPAG16 [132]. Similarly, arsenic trioxide inhibited the expression of the DDX25 and CRM1 mRNAs, as well as the downstream proteins HMG2, PGK2, and H4 necessary for spermatogenesis in mice [133]. Oral exposure to arsenic trioxide at doses of 0.3 and 30 µg/kg for 15 days resulted in a dose-dependent frequency of sperm production in mice with aberrant head morphology [134]. Diabetic rats exposed to sodium arsenite at a concentration of 10 mg/L in drinking water for 40 days caused a drop in serum testosterone, sperm counts, motility, morphology, and acrosomal and plasma membrane structure [135]. Decreases in sperm count and viability with increased arsenic accumulation, lipid peroxidation, and protein carbonylation in the testes have been observed by administration of sodium arsenate at a concentration of 10, 25, 50, 100, and 200 ppm for 40 days through drinking water in mice [136]. Rats exposed to sodium arsenite at doses of 1, 5, and 25 mg/L through drinking water for 6 months showed compromised sperm counts and motility, and testosterone and altered 19 proteins related to reproduction such as Vdac3, Prkaca, Hspa41, Spaca1, Ma1b, Gpx4, Safb1, Trim28, Rbp1, Hsd11b1, Mapk3, Gpd2, Ace, Hspa11, Dnaja1, Ybx3, Smcp, Nasp, and Cabs1 were altered [104].
6. Pre-Natal Arsenic Exposure Induced Male Reproductive Toxicity in Animals
The growing evidence suggests that parental and/or pre-natal arsenic exposure to animals resulted in post-natal developmental toxicity. In-utero exposure to sodium arsenite from embryonic day-10 to day-18 at a concentration of 10 ppb induced an increase in leptin levels, and at 42.5 ppm reduced the litter size compared to control mice [137]. Chronic As trioxide exposure to parental male at a dose of 1 mg/L showed genotoxic damage in F0-F3, altered methylation patterns, changes in reproductive parameters, morphological damage in the ovaries (F0 and F1) and testicles (F1–F3), and compromised sperm quality (F0-F3, except F2) [138]. Exposure of sodium arsenite at the dose of 10 mg/L in drinking water to pregnant females from GD1-21 affected body weight and initial sexual development in male pups and relative anogenital distance also showed changes in the expression of SOD1, SOD2, CAT, and GSTK1 gene in male pup rat [139]. Administration of sodium arsenite at a concentration of 10 mg/L showed lower sperm production, sperm count, motility, and quality in the epididymis of rats [140]. Daily sperm production and the number of spermatids in the rat epididymis were reduced after oral treatment of sodium arsenite at doses of 0.01 and 10 mg/L for 56 days [141]. Lead at the dose of 819 mg/L was exposed to pregnant rats through drinking water until weaning, followed by the exposure of arsenic to male offspring at a dose of 2.3 mg/L, which showed a decrease in daily sperm production, relative weights of testes, epididymis, seminiferous tubules, and prostate, and decreased the activity of 3β-HSD and 17β-HSD in male offspring [142]. Sodium fluoride and sodium arsenite at 100 mg/L and 50 mg/L, respectively, via drinking water during the pre-pregnancy period, decreased the testicular weights, serum FSH, LH, and testosterone levels, and increased Beclin1 and LC3 expressions in Sprague Dawley (SD) rats’ testes [143].
This entry is adapted from the peer-reviewed paper 10.3390/toxics10120744
This entry is offline, you can click here to edit this entry!
