Actuators generate motion. Skeletal muscle, as the best known bio-actuators driving bio-movement in nature, consists of bundled fascicles that can further break down into highly contractile units called myofibrils [
1]. Blood vessels and motor neurons are integrated inside to regulate metabolite circulation and bioelectrical transport [
2]. This muscle–vessel–neuron trinity includes three core elements of actuation, power, and control, a principle human-made actuators follow. Among the three, the choice of actuator seems less challenging comparing to the other two, given the apparent value of high-performance tactile sensors for dexterous robotics [
3,
4], and the value of lightweight powering for aerosol insect-mimic flying robotics [
5,
6,
7]. However, most actuators reported to data are designed for ex vivo applications and may fail to undertake tasks in vivo due to mechanical, biochemical, or thermal mismatch with the surrounding bio-ambient conditions.
The past decade has seen a rapidly expanding library of biomedical actuators in piezoelectric [
8,
9], electro-/thermo-active [
10,
11], magnetic, and pneumatic materials [
12,
13]. Each category provides technical advantages for certain applications. For example, pneumatic actuators can generate a large strain above 300% but only work at a low frequency [
14]. By contrast, electro-actuators can provide frequency over 1 kHz for high-speed actuation [
15]. In addition to the technical specifications of the actuator itself, the selection of an actuator involves considerations of power and control. For instance, implementing pneumatic and hydraulic actuations in the body requires a water or air supply via a catheter or cable, yet magnetic or electric actuators can be powered in a completely cable-free approach. Moreover, choosing an actuator for in vivo applications often involves the consideration of additional aspects including durability and biocompatibility with the surrounding tissues [
16,
17,
18]. Most of the existing reviews focus on one subcategory such as pneumatic, electroactive, or optical actuators [
19,
20,
21,
22,
23], and their applications in drug delivery, thrombus removal, or ventricular assist devices (VADs). Readers often need to go through each subcategory to acquire a full vision for selecting an actuator. For example, the blood-circulating pump in VADs usually consist of battery-powered motors and rotors. Seeing a good match with respect to the stress and frequency of the heart, Roche et al. developed a soft VAD based on creative use of pneumatic actuators [
24], which has been leveraged in seemingly non-relevant applications such as rehabilitation [
25,
26] and gastric surgery [
27,
28]. We believe a broad view of emerging actuating solutions could be more efficient for researchers to narrow the choice down to a best fit.
2. Actuators for Various In Vivo Biomedical Applications
The four mega categories of in-body actuator applications are cardiovascular devices, endoscope and surgery-assistant devices, drug-delivery devices, and micro-swimmers (
Figure 1). Additionally, there are emerging materials and strategies that are likely to foster future implementations even the current prototypes remain preliminary.
Figure 1 provides a landscape of promising actuators for in-body uses, where drug delivery and cardiac/cardiovascular occur as two major subfields. Actuators used in GI capsule robots/patches can be as simple as springs or balloons, yet there are challenges on auto-triggering designs. To address this, various methods have been explored, such as pH-sensitive or glucose-responsive coatings [
34]. Hydraulic actuators are preferred for endoscopes as fluid lines are typically embedded in existing endoscopes. Conventional brush or brushless motors remain the primary locomotive solution for the best control of forward/reverse camera motions despite emerging options such as magnetic and micromotors. Operating a magnetic drug-delivery device often requires an external magnet to drive the vehicle toward the target area, typically assisted by a monitor to track and guide its in-body locomotion [
35,
36]. This scenario implies magnetic and hydraulic actuator-based devices are ideal for surgical-assistant tools or in-clinic drug administrations monitored by healthcare professionals [
37,
38,
39,
40].
Figure 1. Actuators in various biomedical devices for major in-body applications [
24,
37,
38,
39,
40,
41,
42,
43,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73].
2.1. Actuators for Cardiac and Cardiovascular Devices
The heart is the major in-body actuator supporting oxygen supply and metabolites. The Worldwide prevalence of heart failure (HF) is 63.34 million cases, accounting for a total global financial cost estimated at $346.17 billion per year [
74]. HF patients suffer insufficient blood flow due to reduced ventricular function, which is caused by either incomplete muscle squeeze or decreased ventricle volumetric capacity [
75]. Repairing cardiac dysfunction by leveraging artificial soft actuators is conceptually straightforward, and the related engineering efforts are particularly valuable for patients before heart translation. One unfortunate fact is that many HF patients on the waiting list die from end-stage heart dysfunction because donor organ availability is far below the need [
76]. As one alternative rescue solution, ventricular assistance devices (VADs) have been developed as a bridge until transplantation is conducted or as a permanent solution to restore cardiac functions in case of persistent organ shortage [
77].
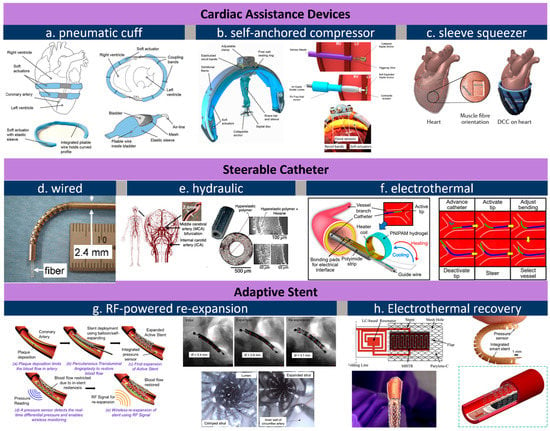
From the inside to the outside of the heart, external VADs aim at direct cardiac compression for augmenting blood flow [
78]. As an artificial muscle layer, these devices assist ventricular compression without contacting blood, which forgoes the need for blood-thinning agents and their associated risks. Moreover, external VADs can be placed away from coronary vessels and other risky sites. One McKibben-based pneumatic VAD cuff was developed by the Walsh Group and tested in vivo, shown as
Figure 2a [
41]. Their observations confirmed that reliable device–tissue interfacing and the appropriate tuning of the contraction rate are critical to an optimal cardiac output. Upon being coupled with the heart, a systolic period of 40% achieves the highest aortic flow rate of 2 L min
−1. The results also suggest an improved refilling function of the heart during diastole. Its pneumatic pumping and control can follow the convenient method of the existing FDA-approved pneumatic SynCardiaTM system [
79]. Shortly after, the team attempted to address the device-tissue coupling problem by adding an inflatable anchor clamping the interventricular septum, shown in
Figure 2b [
42]. The bracing assembly consisted of a bracing bar that passes through a ventricle wall and a semilunar bracing frame around the ventricle with the integration of pneumatic actuators. In this setting, force sensors are included to provide real-time monitoring of compression forces, a critical leap toward soft VAD robots. Their next advance is a soft robotic sleeve that combines compressions and twists (
Figure 2c) [
24]. This combined actuation mechanism mimics the operation of a natural heart where layers of multiple linear contractile filaments are orientated along helical and circumferential patterns [
80,
81]. The biomimetic sleeve can be equipped with a control system designed to synchronize its actuation with the native cardiac cycle. This allows the fine-tuning of the output force and the timing of disease-specific needs. Pneumatic actuators in the devices above leverage a wall-compressed air supply for actuation. Ongoing efforts are focused on wearable pumping and control so that the on-heart actuator can work in a similar way to a more mature device [
78]. Size, weight, biocompatibility, and durability are among the major remaining challenges that are currently preventing its practical use [
82,
83]. Nevertheless, from a more positive perspective, we should underscore that the power and control system required here are no more complex compared to the existing VADs, and that its improved mechanical match with tissues at a more reasonable cost proves unreplaceable by mechanical actuators.
Figure 2. Actuators for cardiovascular applications. (
a), pneumatic cuff that is wired to heart’s contour [
41]; (
b), a self-anchored assistance-squeezer [
42]; (
c), a pneumatic sleeve for twist-n-squeeze actuation [
24]; (
d), steerable catheter through wired control [
44]; (
e), a fluid-steered catheter [
45]; (
f), electrothermally controlled steering of catheter tip [
43]; (
g), RF-triggered re-expansion against restenosis concept (left) and its in vivo image before and after RF expansion (right) [
87]; (
h), electrothermal treatment of restenosis [
89]. All images are reproduced or adapted with permission.
The dimension for an actuator drops below the millimeter level from the heart to vessels. Medical catheters are widely used for endovascular surgeries treating illnesses such as cerebral aneurysms [
14,
84]. Actuators for catheters are born with a genetic advantage because the control/power stimuli (e.g., hydraulic or pneumatic) can be delivered along the catheter itself. One major challenge is that the tips of conventional catheters generally lack dexterity and are typically operated by skilled healthcare professionals under X-ray fluoroscopy [
43]. To address this issue, manually operated wires [
44] can be embedded in the catheter wall so that the bending can be controlled by an external electromechanical device (
Figure 2d). Replacing wires with water pressure, a hydraulically steerable catheter (
Figure 2e) can be embedded with four 50 μm wide fluid sub-channels that are uniformly arranged in the wall of a 0.9 mm-diameter catheter [
45]. Infilling one channel triggers an expansion and bends the catheter toward the opposite side; in a similar regime, infilling two adjacent channels results in a combined bending at 45°. As such, the usability of such a configuration has been verified through tortuous cerebral vasculature and by deploying coils, and the catheter successfully accessed the ascending pharyngeal artery and parotid artery in ex vivo studies. The electrothermal input is another type of stimulus used for steering control. Selvaraj et al. recently developed a proof-of-concept catheter based on thermal-responsive hydrogel [
43]. Here, repetitive bending is controlled by heating an integrated planar copper coil at the 5 mm-wide free end (
Figure 2f). Note that at room temperature the catheter tip is fully curved. Specifically, bending is triggered at a critical temperature, around 28–32 °C; at a power of 3.5–4 W; and reaches a bending angle of 170° at 50 °C. To prevent the influence of body temperature, the tip needs to be encapsulated in thermal insulation. Bilateral bending remains a challenge for this design as only one-side is attached to the heating coil. Furthermore, the down-scaling of this device is feasible when the critical temperature can be tuned slightly below body temperature so the tip can hold a desired deformation to save power.
Stenting is a common solution to severe atherosclerosis caused by progressive plaque buildup on the arterial wall. Restenosis is one leading cause of stent dysfunctionality and the requirement of surgical intervention. Integrated pressure sensors and remote stent heating have been reported to detect and prevent restenosis in vivo [
85,
86], while controllable re-expansion proves an effective route to eliminate vessel re-narrowing risks. Shape memory alloy (SMA), as a thermoactivated material, induces re-expansion when the stent’s resonant frequency matches an external RF trigger signal [
46,
87]. In the example shown in
Figure 2g, a 2 mm-diameter nitinol SMA stent expanded to 3.2 mm in diameter under 11.7 W RF of power at 315 MHz within 220 s, or re-expanded to 4.2 mm under 29.5 W in 100 s. The large, controllable expansion suggests its usability as a durable implant without re-intervention or re-stenting procedures. Moreover, thermal actuation based on wireless heating proves to be effective to mitigate hyperthermia as well (
Figure 2h), where temperature and force sensors offer closed-loop manipulation of smart stents [
88,
89].
2.2. Actuators for Endoscope and Surgery Assistance
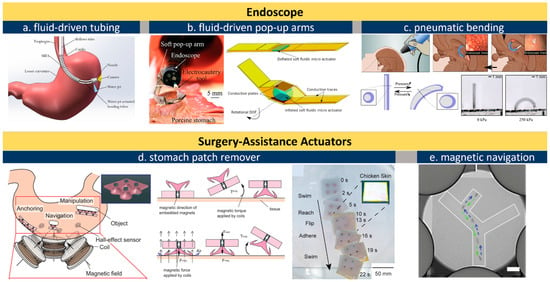
Endoscopes are wired or wireless GI devices that provide combined capabilities of image capturing, biopsy sampling, and surgical interventions [
50]. Wired endoscopes can be inserted from natural orifices such as the rectum or mouth. The cable is typically in the range of 8–12 mm in diameter and consists of multiple sub-channels of optical or electric paths as well as fluid lines for camera flushing and GI tract inflation. An external controller with knobs is maneuvered by skilled professionals for steering, flushing, and imaging (
Figure 3a). Conventional miniatured actuators based on micro-electromechanical systems (MEMS) are used for tissue sampling and surgical purpose but this often requires operation at a high voltage or temperature [
90]. Russo et al. addresses this challenge by developing a low-cost fluid-driven robotic arm that enables safe interaction with surrounding tissues, shown in
Figure 3b [
48]. The team proposed a “soft pop-up regime” to offer sufficient force and gentle interaction with GI tissues. Their design leverages existing fluid lines to inflate/deflate a hemispherical microballoon joint for soft arm operation. One engineering advantage is that this soft fluidic microactuator (SFMA) can be fabricated in large batches [
91]. Endoscope manipulation can also be assisted by an pneumatic actuator, shown in
Figure 3c, which offers better mechanical matches with tissues thereby reducing the risk of accidental damage [
92].
Figure 3. Actuators for endoscopes. (
a), schematic illustration of the fluid line in a wired endoscope kit [
110]; (
b), an endoscope equipped with soft pop-up arms [
44,
48]; (
c), pneumatically inflated actuation of intracranial endoscope [
92]; (
d), a magnetic soft vacuum sucker to remove surgery patch in stomach [
106]; (
e), X-ray navigation of magnetic actuators under skin [
102]. All images are reproduced or adapted with permission.
To avoid wiring issues, wireless capsule endoscopes (WCE) have been developed toward convenience operations and patient comfort since 2000 [
93]. Unlike tethered probes, catheters, and endoscopes that struggle to reach the small intestine, WCE run through the entire GI tract with minimum human intervention and discomfort for minimally invasive diagnosis of unknown abdominal pain, GI hemorrhages, small bowel tumors, and Crohn’s disease [
94]. The revolutionary features above make WCE a gold standard as small-intestine endoscopes where biopsies and active locomotion are not desired. A WCE passes through the GI tract within 24 h, captures images at a frequency of 4–6 frames per second, and transmits data to a wearable recorder via electric-field propagation or radio-frequency connection [
95,
96]. Despite features above, one major limitation of conventional WCEs is the absence of controllable locomotion. This has spawned explorations in advanced wireless actuation, which in turn fosters the evolution of in-gut robots. As to the choice of actuator, brushed/brushless DC motors have been leveraged for actuating legs and propellers in a WCE for forward and backward motion [
47,
49,
97]. Adding one on-board magnetic actuator and then navigating the capsule with an external magnet can accelerate the capsule’s motion, which renders a favorable combination of rapid locomotion and precise actuation of robotic arms [
98] for facile tissue manipulation. Sensors including pH, pressure, temperature, and gas-molecule detectors can be further integrated in such a capsule [
99,
100], updating it into a multifunctional robotic platform.
Actuators can provide surgical assistance under wired or wireless control. The use of an electromagnetic field is a widely reported approach to operate magnetic actuators in the GI tract. The orientation and magnitude of the external magnetic field can be controlled, and accordingly, in-body microrobots can be manipulated in closed spaces by loading X-ray contrast agents (e.g., Lipiodol) in micro-actuators [
101]. Lipiodol-loaded, visualized microrobots allow easy targeting and retrieval owing to the hydrophobic properties of the Lipiodol agent, and one example is shown in
Figure 3e [
102]. Moreover, being observable enables flexible actuator manipulations including rotation, lifting, and flipping. The fine motion control of magnetic actuators makes it possible to precisely locate and even tune the force exerted on the inner wall of the intestine [
40], uterus [
103], and stomach [
104,
105]. Hwasaki et al. reported a soft patch remover driven by a magnetic actuator, shown in
Figure 3d [
106]. The remover is navigated to the target (stomach patch) and compressed firmly against the target to create negative pressure (pseudo-vacuum). Next, one side of the patch is lifted by the suction cup to peel it off. This combination of magnetic actuators and X-ray or ultrasound-imaging techniques proved an effective approach to press microneedles into the wall of the intestine and uterus for drug delivery, embryo transfer, and tumor surgeries, the details for which can be found in recent reviews on magnetically controlled soft robots [
107,
108,
109].