Atmospheric pressure chemical ionization (APCI) is an ionization method used in mass spectrometry which utilizes gas-phase ion-molecule reactions at atmospheric pressure (105 Pa), commonly coupled with high-performance liquid chromatography (HPLC). APCI is a soft ionization method similar to chemical ionization where primary ions are produced on a solvent spray. The main usage of APCI is for polar and relatively less polar thermally stable compounds with molecular weight less than 1500 Da. The application of APCI with HPLC has gained a large popularity in trace analysis detection such as steroids, pesticides and also in pharmacology for drug metabolites.
- chemical ionization
- soft ionization
- liquid chromatography
1. Instrument Structure
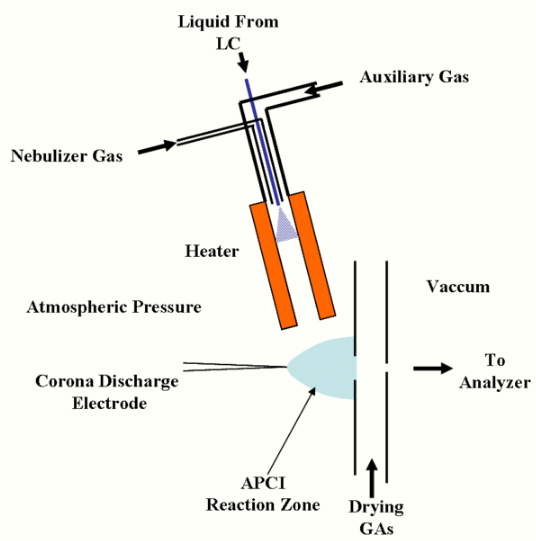
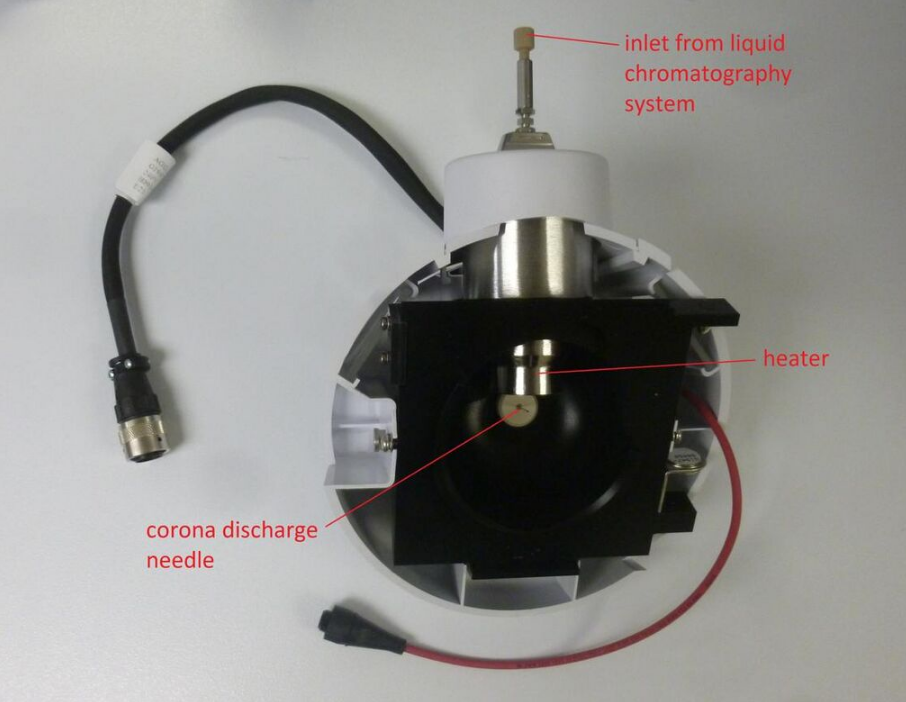
A typical APCI source usually consists of three main parts: a sample inlet, a corona discharge needle, and an ion transfer region under intermediate pressure.[1] In the case of the heated nebulizer inlet[2] from an LC, as shown in the figure, the eluate flows at 0.2 to 2.0 mL/min into a pneumatic nebulizer which creates a mist of fine droplets. Droplets are vaporized by impact with the heated walls at 350-5000 C and carried by the nebulizer gas and an auxiliary gas into the ion molecule reaction region between the corona electrode and the exit counter-electrode.[3] A constant current of 2 - 5 microamps is maintained from the corona needle. Sample ions are produced by ion-molecule reactions (as described below), and pass through a small orifice or tube into the ion transfer region leading to the mass spectrometer.
Various geometries of ion source are possible, depending on application. When used with liquid chromatography, particularly at higher flow rates, the nebulizer is often positioned orthogonal to (or at a similarly steep angle to) the inlet of the mass spectrometer, so that solvent and neutral material does not contaminate the actual inlet of the mass spectrometer.[4]
2. Ionization Mechanism
Ionization in the gas phase by APCI follows the sequences: sample in solution, sample vapor, and sample ions. The effluent from the HPLC is evaporated completely. The mixture of solvent and sample vapor is then ionized by ion-molecule reaction.[5]
The ionization can either be carried out in positive or negative ionization mode. In the positive mode, the relative proton affinities of the reactant ions and the gaseous analyte molecules allow either proton transfer or adduction of reactant gas ions to produce the ions [M+H]+ of the molecular species.[3] In the negative mode, [M−H]− ions are produced by either proton abstraction, or [M+X]− ions are produced by anion attachment. Most work on the APCI-MS analysis has been in positive mode.
In the positive mode, when the discharge current of corona discharge is 1-5 μA on the nebulized solvent, N2 gas molecules are excited and ionized, which produce N4+*. The evaporated mobile phase of LC acts as the ionization gas and reactant ions. If water is the only solvent in the evaporated mobile phase, the excited nitrogen molecular ions N4+* would react with H2O molecules to produce water cluster ions H+(H2O)n.[6] Then, analyte molecules M are protonated by the water cluster ions. Finally, the ionization products MH+(H2O)m transfer out from the atmospheric-pressure ion source. Declustering (removal of water molecules from the protonated analyte molecule) of MH+(H2O)m takes place at the high vacuum of the mass analyzer.[7] The analyte molecule ions detected by MS are [M+H]+. The chemical reactions of ionization process are shown below.
Primary and secondary reagent ion formation in a nitrogen atmosphere in the presence of water:[7][8]
- N2 + e → N2+ + 2e
- N2+* + 2N2 → N4+* + N2
- N4+ + H2O → H2O+ + 2N2
- H2O+ + H2O → H3O+ + OH•
- H3O+ + H2O + N2 → H+(H2O)2 + N2
- H+(H2O)n-1 + H2O + N2 → H+(H2O)n + N2
Ionization of product ions:[7]
- H+(H2O)n + M → MH+(H2O)m + (n-m)H2O
Declustering in the high vacuum of the mass analyzer:[7]
- MH+(H2O)m → MH+ + mH2O
If the mobile phase contains solvents with a higher proton affinity than water, proton-transfer reactions take place that lead to protonated the solvent with higher proton affinity. For example, when methanol solvent is present, the cluster solvent ions would be CH3OH2+(H2O)n(CH3OH)m.[7] Fragmentation does not normally occur inside the APCI source. If a fragment ion of a sample is observed, thermal degradation has taken place by the heated nebulizer interface, followed by the ionization of the decomposition products.
In a major distinction from chemical ionization, the electrons needed for the primary ionization are not produced by a heated filament, as a heated filament cannot be used under atmospheric pressure conditions. Instead, the ionization must occur using either corona discharges or β- particle emitters, which are both electron sources capable of handling the presence of corrosive or oxidizing gases.[3]
3. History
The origins of atmospheric pressure chemical ionization sources combined with mass spectrometry can be found in the 1960s in studies of ions in flames[9] and of ion chemistry in corona discharges up to atmospheric pressure.[10] The first application of APCI combined with mass spectrometry for trace chemical analysis was by the Franklin GNO Corporation who in 1971 developed an instrument combining APCI with ion mobility and mass spectrometry[11]. Horning, Carroll and their co-workers in the 1970s at the Baylor College of Medicine (Houston, TX) demonstrated the advantages of APCI for coupling gas chromatography (GC)[12] and liquid chromatography (LC)[13] to a mass spectrometer. High sensitivity and simple mass spectra were shown in these studies.[13] For LC-MS, the LC eluate was vaporized and ionized in a heated metal block. Initially, a 63Ni foil was used as a source of electrons to perform ionization. In 1975, a corona discharge electrode was developed, providing a larger dynamic response range.[14] APCI with the corona discharge electrode became the model for modern commercially available APCI interfaces.[15]
In the late 1970s an APCI mass spectrometer system (the TAGA, for Trace Atmospheric Gas Analyzer), mounted in a van for mobile operation, was introduced by SCIEX,[16][17] providing high sensitivity for monitoring polar organics in ambient air in real time. In 1981 a triple quadrupole mass spectrometer version was produced, allowing real-time direct air monitoring by APCI-MS/MS. A similar platform was used for the SCIEX AROMIC system (part of the CONDOR contraband detection system developed together with British Aerospace) for the detection of drugs, explosives and alcohol in shipping containers at border crossings, by sampling the interior airspace.[18][19]
In the mid-1980s and into the early 1990s , the advantages of performing LC/MS with APCI and with electrospray, both atmospheric pressure ionization techniques, began to capture the attention of the analytical community.[20] Together they have dramatically expanded the role of mass spectrometry in the pharmaceutical industry for both drug development and drug discovery applications. The sensitivity of APCI combined with the specificity of LC-MS and LC-MS/MS often makes it the method of choice for the quantification of drugs and drug metabolites.[21]
4. Advantages
Ionization of the substrate is very efficient as it occurs at atmospheric pressure, and thus has a high collision frequency. Additionally, APCI considerably reduces the thermal decomposition of the analyte because of the rapid desolvation and vaporization of the droplets in the initial stages of the ionization.[3] This combination of factors most typically results in the production of ions of the molecular species with fewer fragmentations than many other ionization methods, making it a soft ionization method.[22]
Another advantage to using APCI over other ionization methods is that it allows for the high flow rates typical of standard bore HPLC (0.2-2.0 mL/min) to be used directly, often without diverting the larger fraction of volume to waste. Additionally, APCI can often be performed in a modified ESI source.[23] The ionization occurs in the gas phase, unlike ESI, where the ionization occurs in the liquid phase. A potential advantage of APCI is that it is possible to use a nonpolar solvent as a mobile phase solution, instead of a polar solvent, because the solvent and molecules of interest are converted to a gaseous state before reaching the corona discharge needle. Because APCI involves a gas-phase chemistry, there is no need to use special conditions such as solvents, conductivity, pH for LC. APCI appears to be more versatile LC/MS interface and more compatible with reversed-phase LC than ESI.[22]
5. Application
APCI is suited for thermal stable samples with low to medium (less than 1500Da) molecular weight, and medium to high polarity. It is particularly useful for analytes that are not sufficiently polar for electrospray. The application area of APCI is the analysis of drugs, nonpolar lipids, natural compounds, pesticides and various organic compounds, but it is of limited use in the analysis of biopolymers, organometallics, ionic compounds and other labile analytes.[24]
The content is sourced from: https://handwiki.org/wiki/Chemistry:Atmospheric-pressure_chemical_ionization
References
- Dass, Chhabil (2007). Fundamentals of Contemporary Mass Spectrometry. John Wiley & Sons, Inc.. pp. 47. ISBN 978-0-471-68229-5.
- Thomson, BA (2007). Gross, Michael. ed. Encyclopedia of Mass Spectrometry Vol 6. Elsevier. pp. 366–370. ISBN 9780080438016.
- Edmond de Hoffmann; Vincent Stroobant (22 October 2007). Mass Spectrometry: Principles and Applications. Wiley. ISBN 978-0-470-51213-5. https://books.google.com/books?id=6D_Zz2cvgvUC.
- Siuzdak, Gary (1 April 2004). "An Introduction to Mass Spectrometry Ionization: An Excerpt from The Expanding Role of Mass Spectrometry in Biotechnology". Journal of the Association for Laboratory Automation 9 (2): 50–63. doi:10.1016/j.jala.2004.01.004. https://journals.sagepub.com/doi/full/10.1016/j.jala.2004.01.004. Retrieved 21 April 2022.
- AP, BRUINS (1994-01-01). "ATMOSPHERIC-PRESSURE-IONIZATION MASS-SPECTROMETRY .2. APPLICATIONS IN PHARMACY, BIOCHEMISTRY AND GENERAL-CHEMISTRY" (in en). TrAC Trends in Analytical Chemistry 13 (2). ISSN 0165-9936. http://www.rug.nl/research/portal/publications/atmosphericpressureionization-massspectrometry-2-applications-in-pharmacy-biochemistry-and-generalchemistry(2ccf8862-7a54-4af4-88a6-6b75ee997009).html.
- Gates, Paul. "Atmospheric Pressure Chemical Ionisation (APCI)". http://www.chm.bris.ac.uk/ms/theory/apci-ionisation.html. .
- Niessen, Wilfried (2006). Liquid Chromatography Mass spectrometry. Boca Raton, FL: CRC Press, Taylor and Francis Group. pp. 249–250. ISBN 978-0585138503.
- Byrdwell, William Craig (2001-04-01). "Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids" (in en). Lipids 36 (4): 327–346. doi:10.1007/s11745-001-0725-5. ISSN 0024-4201. PMID 11383683. https://dx.doi.org/10.1007%2Fs11745-001-0725-5
- Knewstubb, PF; Sugden, TM (1960). "Mass-Spectrometric Studies of Ionization in Flames. I. The Spectrometer and its Application to Ionization in Hydrogen Flames". Proc. R. Soc. Lond. A255 (1283): 520–535. doi:10.1098/rspa.1960.0084. Bibcode: 1960RSPSA.255..520K. https://dx.doi.org/10.1098%2Frspa.1960.0084
- Shahin, MM (1966). "Mass‐Spectrometric Studies of Corona Discharges in Air at Atmospheric Pressure". J. Chem. Phys. 45 (7): 2600–2605. doi:10.1063/1.1727980. Bibcode: 1966JChPh..45.2600S. https://doi.org/10.1063/1.1727980.
- Collins, DC; Lee, ML (2002). "Developments in Ion Mobility-Mass Spectrometry". Analytical and Bioanalytical Chemistry 372 (1): 66–73. doi:10.1007/s00216-001-1195-5. PMID 11939214. https://www.researchgate.net/publication/11427556. "In early 1971, when IMS was referred to as plasma chromatography (PC), the Franklin GNO Corporation developed the first commercial ion mobility spectrometer–mass spectrometer (IMS–MS) and demonstrated its use as a detector for the identification and analysis of trace amounts of oxygenated compounds (i.e. 1-octanol and 1-nonanol).".
- Horning, E. C.; Horning, M. G.; Carroll, D. I.; Dzidic, I.; Stillwell, R. N. (1973-05-01). "New picogram detection system based on a mass spectrometer with an external ionization source at atmospheric pressure". Analytical Chemistry 45 (6): 936–943. doi:10.1021/ac60328a035. ISSN 0003-2700. https://dx.doi.org/10.1021%2Fac60328a035
- Horning, E. C.; Carroll, D. I.; Dzidic, I.; Haegele, K. D.; Horning, M. G.; Stillwell, R. N. (1974-11-01). "Atmospheric pressure ionization (API) mass spectrometry. Solvent-mediated ionization of samples introduced in solution and in a liquid chromatograph effluent stream". Journal of Chromatographic Science 12 (11): 725–729. doi:10.1093/chromsci/12.11.725. ISSN 0021-9665. PMID 4424244. https://dx.doi.org/10.1093%2Fchromsci%2F12.11.725
- Carroll, D. I.; Dzidic, I.; Stillwell, R. N.; Haegele, K. D.; Horning, E. C. (1975-12-01). "Atmospheric pressure ionization mass spectrometry. Corona discharge ion source for use in a liquid chromatograph-mass spectrometer-computer analytical system". Analytical Chemistry 47 (14): 2369–2373. doi:10.1021/ac60364a031. ISSN 0003-2700. https://dx.doi.org/10.1021%2Fac60364a031
- Byrdwell, William Craig (2001-04-01). "Atmospheric pressure chemical ionization mass spectrometry for analysis of lipids" (in en). Lipids 36 (4): 327–346. doi:10.1007/s11745-001-0725-5. ISSN 0024-4201. PMID 11383683. https://dx.doi.org/10.1007%2Fs11745-001-0725-5
- Cappiello, Achille (2007). Advances in LC-MS Instrumentation. Netherlands: Elsevier. pp. 11–26. ISBN 9780444527738. https://books.google.com/books?id=rJAWmOW_HuMC&pg=PA13.
- Thomson, BA; Davidson, WR; Lovett, AM (1980). "Applications of a Versatile Technique for Trace Analysis: Atmospheric Pressure Negative Chemical Ionization". Environmental Health Perspectives 36: 77–84. doi:10.1289/ehp.803677. PMID 6775945. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1637749
- Pasilis, Sofie P.; Van Berkel, Gary J. (2010). "Modern Atmospheric Pressure Surface Sampling/Ionization Techniques". in Trantor, George E.. Encyclopedia of Spectroscopy and Spectrometry. London: John Lindon. pp. 819–829. ISBN 9780080922171. https://books.google.com/books?id=LQTB8eKcMz8C&pg=PT5227.
- Government of Canada, Public Services and Procurement Canada (1991). "On-Site Sampling and Detection of Drug Particles". https://publications.gc.ca/site/archivee-archived.html?url=https://publications.gc.ca/collections/collection_2008/ps-sp/PS63-2-1991-10E.pdf.
- Thomson, Bruce A. (1998-03-01). "Atmospheric pressure ionization and liquid chromatography/mass spectrometry—together at last" (in en). Journal of the American Society for Mass Spectrometry 9 (3): 187–193. doi:10.1016/S1044-0305(97)00285-7. ISSN 1044-0305. Bibcode: 1998JASMS...9..187T. https://dx.doi.org/10.1016%2FS1044-0305%2897%2900285-7
- Taylor, Lester C. E.; Johnson, Robert L.; Raso, Roberto (1995-05-01). "Open access atmospheric pressure chemical ionization Mass spectrometry for routine sample analysis" (in en). Journal of the American Society for Mass Spectrometry 6 (5): 387–393. doi:10.1016/1044-0305(94)00124-1. ISSN 1044-0305. PMID 24214220. https://dx.doi.org/10.1016%2F1044-0305%2894%2900124-1
- Zaikin, Vladimir G.; Halket, John M. (2017). "Derivatization in Mass Spectrometry—8. Soft Ionization Mass Spectrometry of Small Molecules". European Journal of Mass Spectrometry 12 (2): 79–115. doi:10.1255/ejms.798. ISSN 1469-0667. PMID 16723751. https://dx.doi.org/10.1255%2Fejms.798
- Holčapek, Michal; Jirásko, Robert; Lísa, Miroslav (2012). "Recent developments in liquid chromatography–mass spectrometry and related techniques". Journal of Chromatography A 1259: 3–15. doi:10.1016/j.chroma.2012.08.072. ISSN 0021-9673. PMID 22959775. https://dx.doi.org/10.1016%2Fj.chroma.2012.08.072
- Holčapek, Michal; Jirásko, Robert; Lísa, Miroslav (2010-06-18). "Basic rules for the interpretation of atmospheric pressure ionization mass spectra of small molecules". Journal of Chromatography A. Mass Spectrometry: Innovation and Application. Part VI 1217 (25): 3908–3921. doi:10.1016/j.chroma.2010.02.049. PMID 20303090. https://dx.doi.org/10.1016%2Fj.chroma.2010.02.049
