Clothing, one of the basic needs, demands the growth of textile industries worldwide, resulting in higher consumption and pollution of water. Consequently, it requires extensive treatment of textile effluent for environmental protection as well as reuse purposes. Primary treatment, secondary treatment, and tertiary treatment are the three major phases of textile wastewater treatment. Secondary treatment under aerobic and anaerobic circumstances is carried out to decrease BOD, COD, phenol, residual oil, and color, whereas primary treatment is utilized to remove suspended particles, oil, grease, and gritty materials. However, biological treatment is not fully capable of treating water according to discharge/reuse standards. Hence, tertiary treatment is used to remove final contaminants from the wastewater.
1. Introduction
One of the most pressing global concerns that affect environmental stability, ecosystem health, and long-term economic development is meeting the rising demand for water that has resulted from the worldwide expansion of industry [
1,
2,
3,
4]. The industrial sector is responsible for a significant proportion of the world’s total water usage. The textile industry is well-known for producing wastewater that contains significant amounts of wasted dyes and chemicals, which are very harmful to aquatic environments and the lives inside them [
5]. In different stages of textile wet processing operations, water is consumed for sizing/de-sizing, scouring, bleaching, mercerizing, dyeing, printing, and finishing. It has been reported that around 100–200 L of water is needed in the processing of 1 kg of the textile product, depending on the type of process [
6]. Inadequate treatment and subsequent release of this large amount of hazardous chemical-laden water may cause severe aquatic environment contamination, affecting aquatic ecosystems and, consequently, human health [
7]. Therefore, efficient textile wastewater treatment has been prioritized by water experts and environmentalists to operate the textile industry in a sustainable way and avoid jeopardizing nature [
8].
In the last few years, textile wastewater treatment has evolved significantly from its origin [
9]. The first two stages of wastewater treatment, e.g., primary and secondary, were established and standardized by many water experts over the years [
10]. However, the scientific community has put a lot of effort into advancing the third stage or tertiary wastewater treatment process. Earlier, the primary objective of textile wastewater treatment was the safe disposal of wastewater without the concept of reusing and resource recovery [
11]. However, the wastewater treatment perspectives have now shifted towards advanced treatment and subsequent recovery, particularly in regions where water is a scarce resource [
12]. The stringent regulations and legislations worldwide have mandated industries to adopt sustainable water management strategies to reduce water consumption and reclaim water to be reused to minimize environmental impact [
13]. In the wet processing of textiles, there are a large number of distinct wastewater streams, each of which has its own unique properties [
14,
15]. These characteristics are determined by the treated materials, processing techniques, chemicals utilized, and other factors. It has been claimed that roughly 60–90% of the process water is typically utilized for rinsing and washing, which may be readily cleaned and recycled back into the process [
16]. Additionally, the water that is contaminated with dyes, salts, and other chemicals may be treated using a variety of methods, which will lead to an increase in the amount of water that can be reclaimed and the number of chemicals that can be recovered. Because of this, the techniques for treating wastewater and the recovery of resources from wastewater have recently been important areas of study [
17,
18].
Until now, scientists have proposed different tertiary treatment techniques, e.g., adsorption, electrochemical processes, advanced oxidation, and membrane-based filtration, to achieve the highest treatment efficiency [
19,
20]. In different countries, the concept of zero-liquid discharge has revolutionized the wastewater treatment process. Zero-liquid discharge (ZLD) technology’s ability to optimize water recycling while simultaneously lowering wastewater quantities has sparked widespread interest in its potential for reuse and resource recovery [
21]. The ZLD techniques use a closed water cycle to ensure that any water that can be recycled after being properly treated is kept inside the system [
22]. The clean water movement, declining groundwater levels in different countries, enforcement of stringent environmental regulation policies, increasing costs and demand for freshwater, and the rising costs and difficulties associated with wastewater disposal are accelerating efforts toward achieving ZLD [
23,
24].
The ZLD concept has been misunderstood by many researchers over the years in terms of its applicability and usefulness. The ZLD concept does not only deal with water reuse; the recovery of chemicals used in the total process has been an inherent goal of ZLD [
25]. Thus, to achieve the ZLD goal in textile industries, comprehensive and in-depth knowledge of the overall textile wet process is required. Moreover, distinguishing different wastewater treatment techniques is also important for selecting the best treatment combination to satisfy the ZLD agenda.
2. Stages of the Textile Processing Operation and Effluent Characteristics
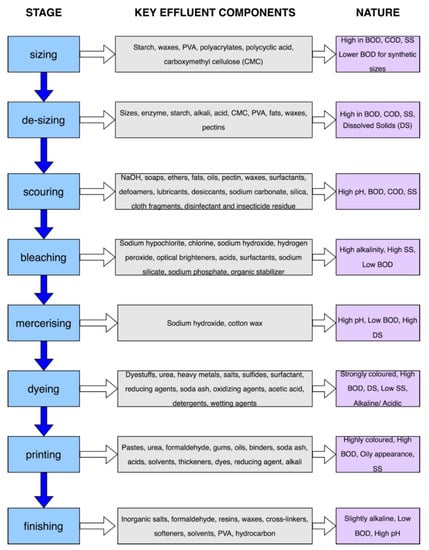
There are both dry and wet processes involved in the manufacturing of fiber in textile mills. The wet processes use a significant amount of water and, as a result, discharge highly polluted effluent. The procedures of sizing, de-sizing, sourcing, bleaching, mercerizing, dyeing, printing, and finishing are included in this step of processing [
26]. This section will provide a concise explanation of these steps in the wet processing of textile materials. In addition, the characteristics of the effluent are shown in
Figure 1, together with the primary components of the effluent that are discharged with the wastewater at each phase. The fibers are given additional strength by the process of sizing, which comes before weaving or spinning [
27]. Sizing ingredients often include things such as starch, polyvinyl alcohol, carboxymethyl cellulose, and similar compounds. A typical textile mill produces around 60,000 square meters of fabric per year, and the wastewater that is released from the mill includes roughly 750 kg of sizing material [
28]. The molecules of dye have a difficult time diffusing into the yarn or fabric due to the presence of the sizing compounds. Before the cloth goes through any further processing, it must first go through a process called desizing, which involves either hydrolysis or oxidation to remove the sizing ingredients. Desizing may be accomplished with the help of enzymes, alkalines, acids, or surfactants [
29]. The oxidation of starch by hydrogen peroxide results in the formation of carbon dioxide and water; enzymes, on the other hand, are responsible for the transformation of starch into ethanol. Scouring is used to remove the different impurities (natural waxes, oils, minerals, pectins, non-cellulosic components, herbicides, pesticides, etc.) that the fibers contain, which obstruct dyeing and finishing. Less scouring is needed for synthetic fibers than for cotton or wool. Generally, hot alkali, detergents, soap solutions, etc., are used as scouring solvents [
30,
31].
Figure 1. Wet processing stages with key effluent components and nature of the wastewater discharge from each stage.
Bleaching is a process that is used to eliminate the natural color content of cloth, which results in the fabric taking on a creamier appearance. Cotton and yarn are the most common substrates for its application, but wool and synthetic fibers may also be used on occasion [
32]. Bleaching agents include substances such as hypochlorite, hydrogen peroxide, and peracetic acid, among others; nevertheless, when compared to the other bleaching agents, peracetic acid is the least harmful to the environment [
33,
34]. Following the bleaching process comes the mercerization step, which is performed to add strength, increase luster, and enhance dye absorption. For the mercerizing process, either zinc chloride or a strong caustic soda solution of around 18–24% (which has to be neutralized by a final acid wash) is used. It is possible to recover sodium hydroxide by utilizing a membrane separation or a multiple-effect evaporator, both of which help to minimize the amount of NaOH that is used [
35].
Dyeing refers to the process of imparting color to cloth by treating it with chemicals, often known as dyes and pigments. Natural dye and synthetic dye are the two kinds of dyes that are frequently used in the textile industry. In comparison to natural dyes, synthetic dye is used more often since it is simpler to produce, comes in a wider variety of colors, and does not fade as easily. There is a wide variety of poisonous dyes and compounds that result from their breakdown. In order to improve the amount of dye that is absorbed by the fibers, it is possible to add various chemicals. Some examples of these chemicals are heavy metals, salts, sulfides, surfactants, and formaldehyde. The majority of the dyeing process’s byproducts, including metals, salts, and colors, may be found in wastewater from the textile industry. The dyeing process causes an increase in the electrical conductivity of the material [
36]. This effect is caused by the use of sodium carbonate and salt. When dyes with low fixing qualities are employed, the effluent from dyeing industries becomes increasingly contaminated. For instance, reactive dyes have a smaller fixing range than cotton and viscose, which may be anywhere from 20–50% [
37]. In printing, dyes are added as a thick paste to a selected section of the fabric to build the design. Urea, PVC, phthalates, gums, binders, etc., are used as printing substances. Printing wastewater has a higher concentration of pollutants compared to dyeing wastewater [
9].
The textile finishing process is used to develop definite properties (softening, flameproof, anti-bacterial, mothproof, waterproofing, rotproof, UV protective, etc.) in the fabric. Wastewater from the finishing process is low in volume but can contain toxic substances such as biocides used to provide anti-microbial characteristics, pentachlorophenols, ethylchlorophospahtes, etc. [
38]. Wastewater effluent from different processes of the textile industry shows different characteristics based on fabrics processed, processing ways, chemicals used, etc. These characteristics are very important factors in selecting the treatment process, the dosage of treating chemicals, and many other factors. The effluents produced by the various steps of the textile processing stage are distinct from one another in terms of their composition and properties. In general, each section has produced some wastewater, namely a small portion from the sizing, desizing, scouring, bleaching, printing, and finishing stages. However, a relatively large amount of wastewater is produced in the dyeing stage because of dyestuffs’ recalcitrant nature [
39,
40]. Characteristics of the effluent from each of these stages are presented in
Table 1.
Table 1. Characteristics of effluent from different textile wet processing stages.
| Wet Processing Stages |
| |
Sizing |
De-Sizing |
Scouring |
Bleaching |
Mercerizing |
Dyeing |
Printing |
Ref |
| pH |
7–9.5 |
5.83–8 |
10–13 |
6–11.6 |
5.5–14 |
5–12.5 |
4–9 |
[27,41,42,43,44,45] |
COD
(mg/L) |
- |
4600–15,000 |
1470–8000 |
1149–13,500 |
100–2688.5 |
1100–4600 |
785–49,170 |
[27,41,42,43,44,45] |
BOD
(mg/L) |
600–2500 |
4400–5060 |
100–2900 |
50–1700 |
20–300 |
10–1800 |
400–1800 |
[27,41,42,43,44,45] |
Sulfate
(mg/L) |
- |
- |
68.5 |
76.3 |
- |
224.9–758.7 |
- |
[42] |
Chloride
(mg/L) |
- |
- |
342.4 |
90–516 |
199.5 |
213.3–26,000 |
- |
[27,42,43] |
Copper
(mg/L) |
- |
- |
- |
- |
- |
0.38–0.43 |
- |
[42] |
Chromium
(mg/L) |
- |
- |
- |
- |
- |
0.39–1.23 |
- |
[42] |
TDS
(mg/L) |
- |
8700–10,200 |
6323 |
2400–22,000 |
5000–12,000 |
35,000 |
2000 |
[27,41] |
TS
(mg/L) |
- |
76,000–32,000 |
7600–17,400 |
2300–14,400 |
600–1900 |
500–50,000 |
2500 |
[27,41,44] |
TSS
(mg/L) |
- |
400–4000 |
- |
288.5 |
105.2 |
499.4 |
125–9500 |
[27,43] |
SS
(mg/L) |
240–260 |
200–270 |
55 |
420–6500 |
2200 |
26,000 |
15,000–20,000 |
[41] |
NO3−
(mg/L) |
- |
- |
- |
5.54 |
9.4 |
6.06 |
- |
[41,43] |
NH4+
(mg/L) |
- |
- |
- |
8.0 |
8.53 |
14.34 |
20–370 |
[27,43] |
H2S
(mg/L) |
- |
- |
- |
5.44 |
1.31 |
1.62 |
- |
[43] |
3. Treatment Methods
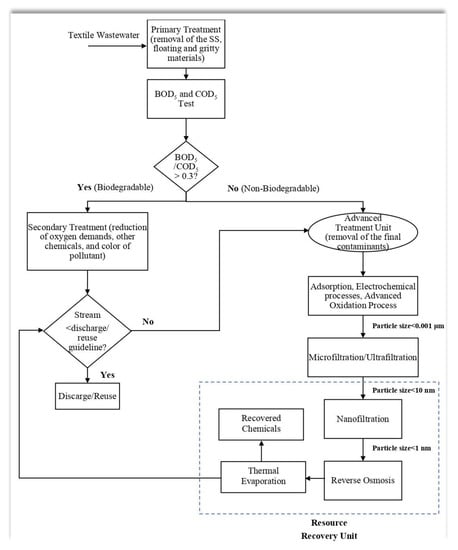
The process of treating wastewater from textile mills typically consists of three core stages: primary, secondary, and tertiary treatment. Throughout each of these processes, various impurities are eliminated, resulting in cleaner water. In general, the primary treatments involve the removal of SS, floating, and gritty materials; the secondary treatments involve the reduction in oxygen demands, other chemicals, and the color of the pollutant; and finally, the tertiary treatments involve the removal of any final contaminants that are still present in the pollutant after the primary and secondary treatments have been completed. In this analysis, numerous techniques for treating the dye in textile wastewater and reducing the pollutant load were addressed. These techniques have the potential to be implemented.
Figure 2 shows a recommended logic diagram to guide the selection of possible treatment methods. BOD
5/COD
5 test should be conducted, followed by primary treatment, to assess the biodegradability criteria. Yukseler et al. [
46] have mentioned the reuse criteria of textile wastewater for different parameters such as COD, color, pH, turbidity, TDS, TSS, etc. Moreover, Katheresan et al. [
47] listed the international standard for dye effluent discharge. Depending on the analysis results, the treated stream can either be recycled as process water towards the manufacturing section or discharged into the environment.
Figure 2. Logic diagram for the selection of wastewater treatment method.
This entry is adapted from the peer-reviewed paper 10.3390/su142215398