Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Hematology
Myelodysplastic syndromes (MDS) are a group of clonal hematological neoplasms characterized by ineffective hematopoiesis in one or more bone marrow cell lineages. Myelodysplastic syndromes with isolated del(5q) constitute the only MDS subtype defined by a cytogenetic alteration.
- myelodysplastic syndromes
- chromosome 5q deletion
- somatic mutations
1. Introduction
Myelodysplastic syndromes (MDS) are a group of clonal hematological neoplasms characterized by ineffective hematopoiesis in one or more bone marrow (BM) cell lineages. Consequently, patients present with variable degrees of cytopenia and dysplasia, which are essential features for establishing a diagnosis according to the World Health Organization (WHO) classification [1,2].
Although nearly half of newly diagnosed patients present a cytogenetic alteration and almost 90% of them harbor at least one somatic mutation, MDS with isolated chromosome 5q deletion (MDS-5q) constitutes the only subtype clearly defined by a cytogenetic alteration [3,4,5,6]. The results of several clinical studies and advances in new technologies have allowed better characterization of this entity. As a consequence, since the first report of the 5q- syndrome, changes and refinements have been made in the definition and characteristics of the patients that pertain to this subtype [7]. Moreover, specific genetic alterations have been found to be associated with prognosis and response to treatments [1,2,8,9].
While preparing this review, the overview of the next WHO classification and the proposal of the International Consensus Classification (ICC) of myeloid neoplasms and acute leukemias were published [2,8]. Consequently, MDS-5q will be renamed and the inclusion criteria will be slightly modified, as will be explained in the subsequent section.
2. From “5q- Syndrome” to MDS-5q
In 1974, Van den Berghe et al. reported a group of three patients with refractory anemia and interstitial deletion of the long arm of chromosome 5. Such cases were later recognized as the “5q- syndrome”. Features of the syndrome included macrocytic anemia, low-normal leukocyte counts, and normal to elevated platelet counts. The BM showed erythroid hypoplasia, hypolobulated megakaryocytes, and a blast count <15% [7]. According to the French–American–British (FAB) cooperative group classification criteria, most of the patients with these characteristics pertain to the group of patients with refractory anemia [10].
It was not until the 2001 edition of the WHO classification that the 5q- syndrome was recognized as a unique and well-defined MDS subtype [11]. In addition to previously described characteristics of this syndrome, in this classification, the blast count threshold was redefined to <5%, and the absence of Auer roads was considered to define 5q- syndrome patients. In 2008, the subtype “MDS with isolated del(5q)” was introduced and the term 5q- syndrome remained restricted to a subset of cases within this category that presented with macrocytic anemia, normal or elevated platelet count, BM erythroid hypoplasia, and a blast count <5% in BM and <1% in peripheral blood (PB) [12]. In the 2017 WHO classification, these cases remained within the MDS with isolated del(5q) subtype. Additionally, the diagnosis of this subtype can be established even if there is one additional cytogenetic abnormality besides the del(5q), unless this abnormality is monosomy 7 or del(7q) [1,13]. This is based on data showing that there is no adverse effect of one chromosomal abnormality in addition to the del(5q) in such patients [14].
As mentioned previously, the overview of the next WHO classification has recently been published [2]. In this new proposal, MDS with isolated del(5q) has been renamed as “myelodysplastic neoplasm with low blast and isolated del(5q)” (abbreviated MDS-5q). The diagnostic criteria have not changed, and it is stated that although an SF3B1 or TP53 mutation (not multi-hit) may potentially alter the biology and/or prognosis of the disease, the presence of such mutations does not per se override the diagnosis of this entity. Regarding the ICC proposal for the classification of MDS, MDS with isolated del(5q) has been retained with no changes from the revised fourth edition of the WHO classification, although the name has been simplified to “MDS with del(5q)”. Similarly to the new WHO proposal, the ICC also specifies that TP53 mutations are admitted in this MDS subtype unless a multi-hit state is detected [8].
It is important to remark that although del(5q) is the most frequent cytogenetic alteration in MDS and is present in roughly 20% of cytogenetic abnormal cases, only about 5% are classified in the MDS-5q category. Features such as higher blast count, alterations in chromosome 7, or a multi-hit TP53 state impact disease prognosis and would reclassify these remaining patients into other, more aggressive, disease subtypes [2,8,15].
The changes in terms and inclusion criteria over time are produced as a consequence of the advances in technology and discoveries, which directly impact our knowledge and the management of this disease (Figure 1).
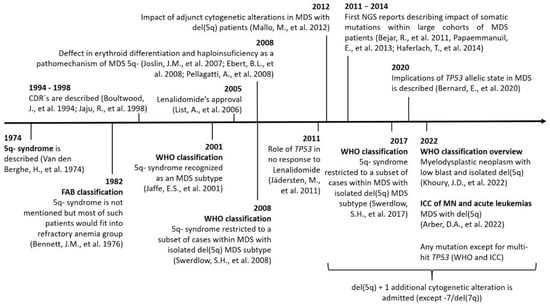
Figure 1. Timeline showing the main discoveries involving MDS-5q and the changes in nomenclature and inclusion criteria. Abbreviations: CDR, commonly deleted region; chr, chromosome; ICC: International Consensus Classification; MN, myeloid neoplasms; NGS, next-generation sequencing.
3. Role of Conventional Cytogenetics in MDS-5q
Conventional cytogenetics (CC) constitutes the gold standard for the genetic diagnosis and prognosis of MDS. However, fluorescence in situ hybridization (FISH) of 5q31 could be useful in cases without evidence of del(5q) by CC. When the presence of MDS-5q is suspected and/or if the cytogenetic study shows no metaphases or an aberrant karyotype with chromosome 5 is involved (no 5q deletion), it is recommended to perform FISH analysis [16,17]. Figure 2 shows the genetic studies available for the diagnosis and characterization of MDS-5q. During follow-up, genetics studies will be adapted to each patient, considering their comorbidities. A new BM aspiration, and the corresponding genetic study, will be performed on suspicion of disease evolution and no response to treatment. In the case of clonal evolution, the approach can be decided according to the general patient status.
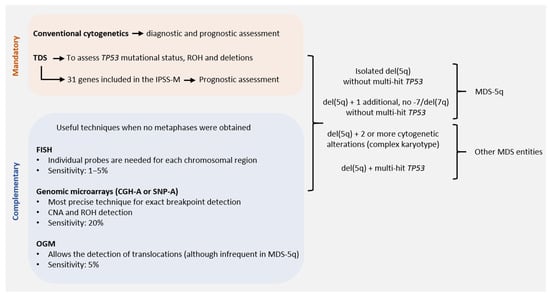
Figure 2. Genetic studies of MDS-5q according to the new diagnostic and prognostic guidelines: techniques available for correct diagnostic and prognostic assessment of MDS-5q according to the criteria of the next World Health Organization (WHO) classification, the proposal of the International Consensus Classification (ICC) and the Molecular International Prognosis Scoring System (IPSS-M). Abbreviations: CGH-A, comparative genomic hybridization; CNA, copy number alteration; FISH, fluorescence in situ hybridization; OGM, optical genome mapping; ROH, region of homozygosity; SNP-A, single nucleotide polymorphism array; TDS, targeted gene sequencing (assuming the use of probes that allow the detection of small CNA and ROH. Otherwise, SNP-A would be recommended to assess CNA and ROH in TP53 for accurate diagnostic and prognostic assessment).
The karyotype is a prognostic variable included in the International Prognostic Scoring System (IPSS) and the revised edition of the IPSS (IPSS-R) [18,19]. Del(5q) alone has always been considered a good prognostic variable and, in the IPSS-R, a concomitant cytogenetic alteration has been included. This change was based on a scoring system proposed by Schanz et al. based on an international data collection of 2902 patients [15]. Deletion 5q is a classical alteration detected in around 15–20% of MDS patients, with half being isolated, around 17% having an additional alteration, and 36% being part of a complex karyotype [3]. The prognostic impact of the accompanying abnormalities in del(5q) is difficult to determine because double abnormalities are highly variable. In 2011, Mallo et al. published an international collaborative study including a large series of del(5q) patients to determine the prognostic impact of adjunct prognostic abnormalities. The multivariate analysis showed that karyotype complexity was one of the main prognostic factors together with platelet count and BM blasts [14]. The good prognosis of del(5q) with one accompanying alteration was included in the MDS with the del(5q) category of the 2017 WHO classification, excluding cases harboring a chromosome 7 alteration [1].
4. Prognostic Impact of Somatic Mutations in MDS-5q
It has been described that one-third of MDS-5q patients present with no somatic alterations, while nearly half of patients (43%) can present with an isolated mutation [35,36]. The pattern of recurrently mutated genes is similar to other MDS subtypes, except for TP53 mutations that were found to be enriched in this subtype of patients [35,37,38]. In the subsequent section, the genes most frequently mutated in MDS-5q are described and Table 1 summarizes their biological and clinical associations and main characteristics and frequencies.
Table 1. Recurrently mutated genes in MDS-5q: clinical and biological correlations.
| Gene | Pathway/Function | Frequency | Clinical and Biological Correlations |
|---|---|---|---|
| SF3B1 | Splicing factor | 19–20% |
|
| DNMT3A | DNA methylation | 18% |
|
| TP53 | Checkpoint/cell cycle | 18% |
|
| TET2 | DNA methylation | 12% |
|
| CSNK1A1 | Proliferation, apoptosis, DNA damage response | 7–10% |
|
| ASXL1 | Chromatin modification | 6% |
|
| JAK2 | Tyrosine kinase | 6% |
|
Abbreviations: AML, acute myeloid leukemia; DTA, DNMT3A, TET2, and ASXL1; OS, overall survival; RS, ring sideroblasts.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14225531
This entry is offline, you can click here to edit this entry!
