To reduce excess soil salinity, plant scientists are employing techniques such as sub-soiling, mixing sand, seed bed preparation, and salt scraping, as well as modern agronomic practices, hydrophilic polymer, gypsum, sulfur acids, green manuring, humic substance, farm yard manures, irrigation system, and salt-tolerant crops. Different organic amendments such as the application of vermi-compost (VC), vermi-wash (VW), biochar (BC), plant growth promoting rhizobacteria (PGPR), and bio-fertilizers (BF) are being used widely to ameliorate the negative consequences of soil salinity. The organic amendments mitigate salt stress via a wide range of mechanisms, including the regulation of ionic homeostasis, antioxidant enzyme activities, and the reduction of oxidative damage. Several studies described that PGPR and BC relieved the negative effects of salinity by increasing the photosynthetic rate, antioxidant enzyme functions, secondary metabolites accumulation, and decreasing ROS in plants. Organic amendments such as VC and VW include a variety of plant growth-regulating components such as micro and macro elements, vitamins, enzymes, and hormones that have been shown to reduce the harmful effects of salts on plants.
- bio-fertilizer
- ionic homeostasis
- organic amendments
- salinity
- vermicompost
1. Vermicompost and Vermiwash
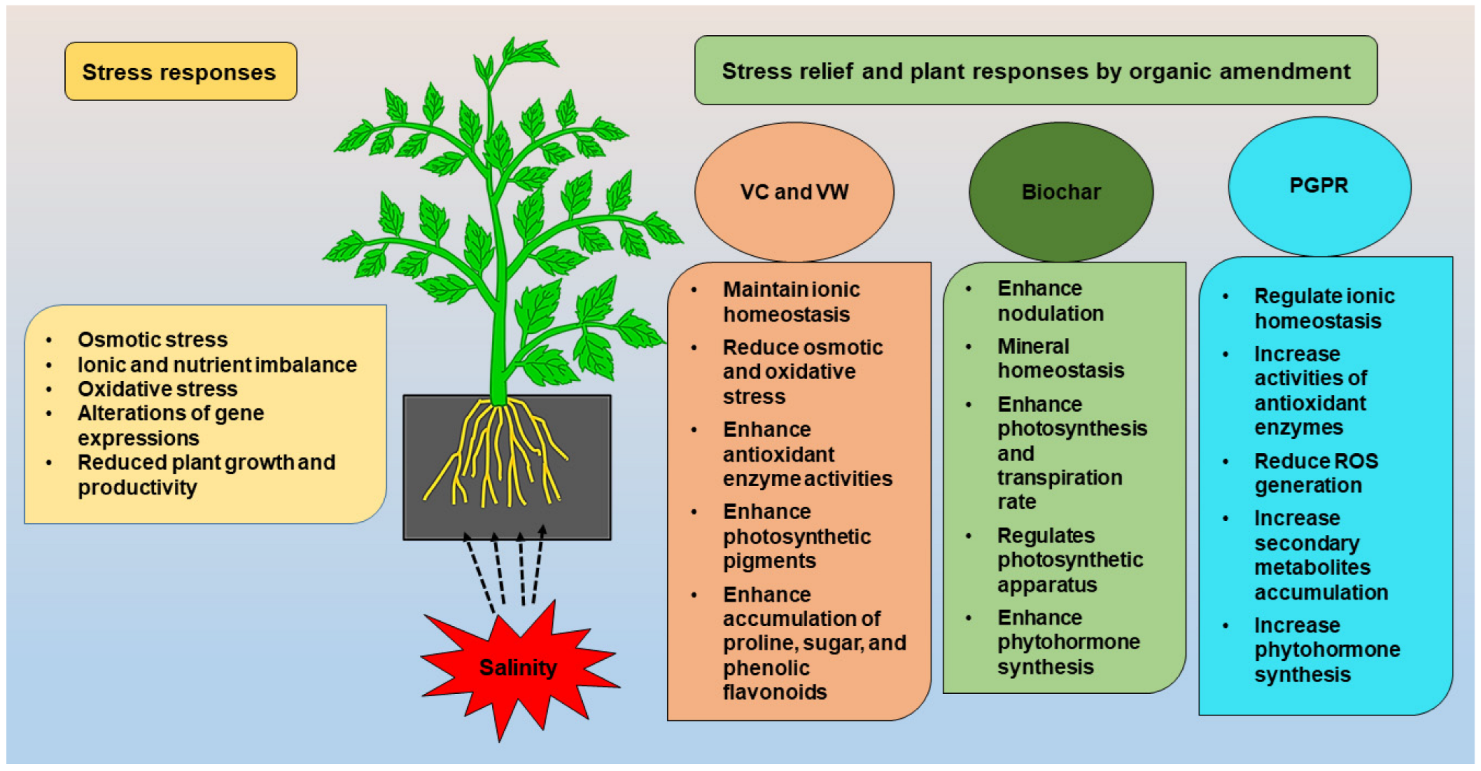
2. Biochar
3. Bio-Fertilizer
4. PGPR
This entry is adapted from the peer-reviewed paper 10.3390/life12101632
References
- Kiyasudeen, K.; Ibrahim, M.H.; Quaik, S.; Ismail, S.A. Vermicompost, its applications and derivatives. In Prospects of Organic Waste Management and the Significance of Earthworms; Springer: Cham, Switzerland, 2016; pp. 201–230.
- Arancon, N.Q.; Lee, S.; Edwards, C.A.; Atiyeh, R. Effects of humic acids derived from cattle, food and paper-waste vermicomposts on growth of greenhouse plants: The 7th international symposium on earthworm ecology · Cardiff · Wales · 2002. Pedobiologia 2003, 47, 741–744.
- Bidabadi, S.S.; Dehghanipoodeh, S.; Wright, G.C. Vermicompost leachate reduces some negative effects of salt stress in pomegranate. Int. J. Recycl. Org. Waste Agric. 2017, 6, 255–263.
- Koozehgar, K.M.; Ardakani, M.R. Effects of vermicomposting and compost tea on nitrogen, phosphorus, and potassium yield and uptake of Mentha aquatic L. inoculated with mycorrhizal fungi Glomus moseae. Iran. J. Plant Physiol. 2017, 11, 10–19.
- Pengkam, C.; Iwai, C.B.; Kume, T. Effects of Vermicompost and Rice Husk Ash on the Change of Soil Chemical Properties and the Growth of Rice in Salt Affected Area. Int. J. Environ. Rural. Dev. 2019, 10, 129–132.
- Demir, Z.; Tursun, N.; Işık, D. Role of different cover crops on DTPA-extractable micronutrients in an apricot orchard. Turk. J. Agric. Food Sci. Technol. 2019, 7, 698–706.
- Ansari, A.A.; Ismail, S.A. Role of earthworms in vermitechnology. J. Agric. Technol. 2012, 8, 403–415.
- Khan, M.H.; Meghvansi, M.K.; Gupta, R.; Veer, V.; Singh, L.; Kalita, M.C. Foliar spray with vermiwash modifies the arbuscular mycorrhizal dependency and nutrient stoichiometry of Bhut jolokia (Capsicum assamicum). PLoS ONE 2014, 9, e92318.
- Nath, G.; Singh, K. Effect of vermiwash of different vermicomposts on the kharif crops. J. Cent. Eur. Agric. 2012, 13, 379–402. Available online: https://hrcak.srce.hr/83274 (accessed on 10 September 2022).
- Beykkhormizi, A.; Abrishamchi, P.; Ganjeali, A.; Parsa, M. Effect of vermicompost on some morphological, physiological and biochemical traits of bean (Phaseolus vulgaris L.) under salinity stress. J. Plant Nutr. 2016, 39, 883–893.
- Chinsamy, M.; Kulkarni, M.G.; Van Staden, J. Garden-waste-vermicompost leachate alleviates salinity stress in tomato seedlings by mobilizing salt tolerance mechanisms. Plant Growth Regul. 2013, 71, 41–47.
- Benazzouk, S.; Djazouli, Z.E.; Lutts, S. Vermicompost leachate as a promising agent for priming and rejuvenation of salt-treated germinating seeds in Brassica napus. Commun. Soil Sci. Plant Anal. 2019, 50, 1344–1357.
- Ahmadi, N.; Akbari, E. The preventive impact of vermicompost on bell pepper (Capsicum annuum L.) salinity resistance: An evaluation. Afr. J. Agric. Res. 2021, 17, 46–56.
- Ebrahimi, M.H.; Taghvaei, M.; Sadeghi, H.; Zarei, M. Effect of organic coats with superabsorbent polymers on improving the germination and early vigor Milk thistle (Silybum marianum L.) seeds under salinity stress. Desert 2019, 24, 207–215.
- Muhie, S.H.; Yildirim, E.; Memis, N.; Demir, I. Vermicompost priming stimulated germination and seedling emergence of onion seeds against abiotic stresses. Seed Sci. Technol. 2020, 48, 153–157.
- Demir, Z. Alleviation of adverse effects of sodium on soil physicochemical properties by application of vermicompost. Compost Sci. Util. 2020, 28, 100–116.
- Barahouee, M.; Sabbagh, E. Influence of vermicompost and salt stress on some characteristics of fenugreek (Trigonellafoenum-graecum L.). Int. J. Agric. Biosci. 2017, 6, 60–63.
- Banadkooki, A.M.; Ardakani, M.D.; Shirmardi, M.; Momenpour, A. Effects of cow manure and vermicompost on growth characteristics of smoke tree (Cotinus coggygria Scop.) under salt stress under greenhouse. Iranian J. Poplar Res. 2019, 26, 483–495.
- Gohari, G.; Mohammadi, A.; Duathi, K.H. Effect of vermicompost on some growth and biochemical characteristic of Dracocephalum moldavica L. under water salinity stress. J. Agric. Sci Sustain. Prod. 2019, 29, 151–168.
- Song, X.; Li, H.; Song, J.; Chen, W.; Shi, L. Biochar/vermicompost promotes Hybrid Pennisetum plant growth and soil enzyme activity in saline soils. Plant Physiol. Biochem. 2022, 183, 96–110.
- Alamer, K.H.; Perveen, S.; Khaliq, A.; Zia Ul Haq, M.; Ibrahim, M.U.; Ijaz, B. Mitigation of salinity stress in maize seedlings by the application of vermicompost and sorghum water extracts. Plants 2022, 11, 2548.
- Bziouech, S.A.; Dhen, N.; Helaoui, S.; Ammar, I.B.; Dridi, B.A.M. Effect of vermicompost soil additive on growth performance, physiological and biochemical responses of tomato plants (Solanum lycopersicum L. var. Firenze) to salt stress. Emir. J. Food Agric. 2022, 34, 316–328.
- Pérez-Gómez, J.D.; Abud-Archila, M.; Villalobos-Maldonado, J.J.; Enciso-Saenz, S.; Hernández de, L.H.; Ruiz-Valdiviezo, V.M.; Gutiérrez-Miceli, F.A. Vermicompost and vermiwash minimized the influence of salinity stress on growth parameters in potato plants. Compost Sci. Util. 2017, 25, 282–287.
- Liu, M.; Wang, C.; Wang, F.; Xie, Y. Maize (Zea mays) growth and nutrient uptake following integrated improvement of vermicompost and humic acid fertilizer on coastal saline soil. Appl. Soil Ecol. 2019, 142, 147–154.
- Oo, A.N.; Iwai, C.B.; Saenjan, P. Soil properties and maize growth in saline and nonsaline soils using cassava-industrial waste compost and vermicompost with or without earthworms. Land Degrad. Dev. 2015, 26, 300–310.
- Jabeen, N.; Ahmad, R. Growth response and nitrogen metabolism of sunflower (Helianthus annuus L.) to vermicompost and biogas slurry under salinity stress. J. Plant Nutr. 2017, 40, 104–114.
- Demir, Z.; Kiran, S. Effect of vermicompost on macro and micro nutrients of lettuce (Lactuca sativa var. Crispa) under salt stress conditions. Kahramanmaraş. Sütçü. İmam. Üniversitesi. Tarım. Doğa. Dergisi. 2020, 23, 33–43.
- Adamipour, N.; Heiderianpour, M.B.; Zarei, M. Application of vermicompost for reducing the destructive effects of salinity stress on tall fescue turfgrass (Festuca arundinacea Schreb. ‘Queen’). J. Soil Plant Interact. Isfahan Uni. Technol. 2016, 7, 35–47.
- Kiran, S. Alleviation of adverse effects of salt stress on lettuce (Lactuca sativa var. crispa) by application of vermicompost. Acta Sci. Pol. Hortorum Cultus 2019, 5, 153–160.
- Hafez, E.M.; Omara, A.E.; Alhumaydhi, F.A.; El-Esawi, M.A. Minimizing hazard impacts of soil salinity and water stress on wheat plants by soil application of vermicompost and biochar. Physiol. Plant 2020, 172, 587–602.
- Yang, A.; Akhtar, S.S.; Li, L.; Fu, Q.; Li, Q.; Naeem, M.A.; He, X.; Zhang, Z.; Jacobsen, S.E. Biochar mitigates combined effects of drought and salinity stress in quinoa. J. Agron. 2020, 10, 912.
- Huang, M.; Zhang, Z.; Zhai, Y.; Lu, P.; Zhu, C. Effect of straw biochar on soil properties and wheat production under saline water irrigation. Agronomy 2019, 9, 457.
- Parkash, V.; Singh, S. Potential of biochar application to mitigate salinity stress in eggplant. Hortc. Sci. 2020, 55, 1946–1955.
- Ibrahim, M.E.H.; Ali, A.Y.A.; Elsiddig, A.M.I.; Zhou, G.; Nimir, N.E.A.; Agbna, G.H.; Zhu, G. Mitigation effect of biochar on sorghum seedling growth under salinity stress. Pak. J. Bot. 2021, 53, 387–392.
- Ibrahim, M.E.H.; Ali, A.Y.A.; Zhou, G.; Elsiddig, A.M.I.; Zhu, G.; Nimir, N.E.A.; Ahmad, I. Biochar application affects forage sorghum under salinity stress. Chil. J. Agric. Res. 2020, 80, 317–325.
- Lashari, M.S.; Ye, Y.; Ji, H.; Li, L.; Kibue, G.W.; Lu, H.; Zheng, J.; Pan, G. Biochar–manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: A 2-year field experiment. J. Sci. Food Agric. 2015, 95, 1321–1327.
- Usman, A.R.; Al-Wabel, M.I.; Abdulaziz, A.H.; Mahmoud, W.A.; EL-Naggar, A.H.; Ahmad, M.; Abdulelah, A.F.; Abdulrasoul, A.O. Conocarpus biochar induces changes in soil nutrient availability and tomato growth under saline irrigation. Pedosphere 2016, 26, 27–38.
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712.
- Jin, F.; Piao, J.; Che, W.; Li, X.; Zhang, C.; Wang, Q.; Hua, S. Peanut shell biochar increases rice yield in highly saline-alkali paddy fields by regulating of leaf ionic concentration and improving leaf photosynthesis rate. Plant Soil 2022, preprint.
- Huang, J.; Zhu, C.; Kong, Y.; Cao, X.; Zhu, L.; Zhang, Y.; Ning, Y.; Tian, W.; Zhang, H.; Yu, Y.; et al. Biochar Application Alleviated Rice Salt Stress via Modifying Soil Properties and Regulating Soil Bacterial Abundance and Community Structure. Agronomy 2022, 12, 409.
- Ekinci, M.; Turan, M.; Yildirim, E. Biochar mitigates salt stress by regulating nutrient uptake and antioxidant activity, alleviating the oxidative stress and abscisic acid content in cabbage seedlings. Turk. J. Agric. For. 2022, 46, 28–37.
- Farhangi-Abriz, S.; Torabian, S. Biochar increased plant growth-promoting hormones and helped to alleviates salt stress in common bean seedlings. J. Plant Growth Regul. 2018, 37, 591–601.
- Nikpour-Rashidabad, N.; Tavasolee, A.; Torabian, S.; Farhangi-Abriz, S. The effect of biochar on the physiological, morphological and anatomical characteristics of mung bean roots after exposure to salt stress. Arch. Biol. Sci. 2019, 71, 321–327.
- Farhangi-Abriz, S.; Torabian, S. Biochar improved nodulation and nitrogen metabolism of soybean under salt stress. Symbiosis 2018, 74, 215–223.
- Hegde, D.M.; Dwivedi, B.S.; Sudhakara Babu, S.N. Biofertilizers for cereal production in India: A review. Indian J. Agric. Sci. 1999, 69, 73–83.
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003, 255, 571–586.
- Khalilzadeh, R.; Seyed, S.R.; Jalilian, J. Growth, physiological status, and yield of salt stressed wheat (Triticum aestivum L.) plants affected by biofertilizer and cycocel applications. Arid. Land Res. Manag. 2017, 32, 71–90.
- Mahmoud, A.A.; Mohamed, H.F. Impact of biofertilizers application on improving wheat (Triticum aestivum L.) resistance to salinity. Res. J. Agric. Biol. Sci. 2008, 4, 520–528.
- Riesty, O.S.; Siswanti, D.U. Effect of biofertilizer on growth and metaxylem diameter of Amaranthus tricolor L. in salinity stress condition. Biogenes. J. Ilm. Biol. 2021, 9, 178–188.
- Khatami, S.A.; Kasraie, P.; Oveysi, M.; Moghadam, H.R.T.; Ghooshchi, F. Mitigating the adverse effects of salinity stress on lavender using biodynamic preparations and bio-fertilizers. Ind. Crops Prod. 2022, 183, 114985.
- Mahdy, A.M.; Nieven, O.F. Interactive effects between biofertilizer and antioxidant on salinity mitigation and nutrition and yield of okra plants (Abelmoschus esculentus L.). J. Soil Sci. Agric. Eng. 2012, 3, 189–205.
- Mahdy, A.M.; Fathi, N.O.; Kandil, M.M.; Elnamas, A.E. Synergistic effects of biofertilizers and antioxidants on growth and nutrients content of corn under salinity and water-deficit stresses. Alex. Sci. Exch. J. 2012, 33, 292–304.
- Souza, J.T.; Cavalcante, L.F.; Nunes, J.C.; Bezerra, F.T.; da Silva, N.J.A.; Silva, A.R.; Oresca, D.; Cavalcante, A.G. Effect of saline water, bovine biofertilizer and potassium on yellow passion fruit growth after planting and on soil salinity. Afr. J. Agric. Res. 2016, 11, 2994–3003.
- AlAbdallah, N.M.; Basalah, M.O.; Roushdy, S.S. The promotive effect of algal biofertilizers on growth and some metabolic activities of (Vigna unguiculata L.) under salt stress conditions. Egypt. J. Exp. Biol. 2017, 13, 187–195.
- El-Shazly, M.; Ghieth, W.M. Effect of some biofertilizers and humic acid application on olive seedlings growth under irrigation with saline water. Alex. Sci. Exch. J. 2019, 40, 263–279.
- de Lima-Neto, A.J.; Cavalcante, L.F.; Mesquita, F.D.; Souto, A.G.; dos Santos, G.P.; dos Santos, J.Z.; de Mesquita, E.F. Papaya seedlings irrigation with saline water in soil with bovine fertilizer. Chil. J. Agric. Res. 2015, 76, 236–242.
- Al-Taey, D.K.; Majid, Z.Z. Study effect of kinetin, bio-fertilizers and organic matter application in lettuce under salt stress. J. Glob. Pharma. Technol. 2018, 10, 148–164.
- Yasmin, H.; Mazher, J.; Azmat, A.; Nosheen, A.; Naz, R.; Hassan, M.N.; Noureldeen, A.; Parvaiz, A.P. Combined application of zinc oxide nanoparticles and biofertilizer to induce salt resistance in safflower by regulating ion homeostasis and antioxidant defence responses. Ecotoxicol. Environ. Saf. 2021, 218, 112262.
- Albdaiwi, R.N.; Khyami-Horani, H.; Ayad, J.Y. Plant growth-promoting Rhizobacteria: An emerging method for the enhancement of wheat tolerance against salinity stress. Jordan J. Biol. Sci. 2019, 12, 525–534.
- Goswami, M.; Deka, S. Isolation of a novel rhizobacteria having multiple plant growth promoting traits and antifungal activity against certain phytopathogens. Microbiol. Res. 2020, 240, 126516.
- Hoque, M.N.; Hannan, A.; Imran, S.; Paul, N.C.; Mondal, M.; Sadhin, M.; Rahman, M.; Bristi, J.M.; Dola, F.S.; Hanif, M.; et al. Plant Growth-Promoting Rhizobacteria-Mediated Adaptive Responses of Plants Under Salinity Stress. J. Plant Growth Regul. 2022, 28, 1–20.
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: Perspectives and challenges. In PGPR Amelioration in Sustainable Agriculture; Woodhead Publishing: Sawston, UK, 2019; pp. 129–157.
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216.
- Gao, Y.; Zou, H.; Wang, B.; Yuan, F. Progress and applications of plant growth-promoting bacteria in salt tolerance of crops. Int. J. Mol. Sci. 2022, 23, 7036.
- Kumawat, K.C.; Sharma, P.; Nagpal, S.; Gupta, R.K.; Sirari, A.; Nair, R.M.; Bindumadhava, H.; Singh, S. Dual microbial inoculation, a game changer?—Bacterial biostimulants with multifunctional growth promoting traits to mitigate salinity stress in Spring Mungbean. Front. Microbiol. 2021, 11, 3491.
- Simonin, K.A.; Burns, E.; Choat, B.; Barbour, M.M.; Dawson, T.E.; Franks, P.J. Increasing leaf hydraulic conductance with transpiration rate minimizes the water potential drawdown from stem to leaf. J. Exp. Bot. 2015, 66, 1303–1315.
- Nawaz, A.; Shahbaz, M.; Imran, A.; Marghoob, M.U.; Imtiaz, M.; Mubeen, F. Potential of salt tolerant PGPR in growth and yield augmentation of wheat (Triticum aestivum L.) under saline conditions. Front. Microbiol. 2020, 11, 2019.
- Singh, R.P.; Jha, P.; Jha, P.N. Bio-inoculation of plant growth-promoting rhizobacterium Enterobacter cloacae ZNP-3 increased resistance against salt and temperature stresses in wheat plant (Triticum aestivum L.). J. Plant Growth Regul. 2017, 36, 783–798.
- Ali, B.; Wang, X.; Saleem, M.H.; Sumaira; Hafeez, A.; Afridi, M.S.; Khan, S.; Zaib-Un-Nisa; Ullah, I.; AmaralJúnior, A.T.D.; et al. PGPR-Mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants 2022, 11, 345.
- Chinnaswamy, A.; Coba de la Peña, T.; Stoll, A.; de la Peña Rojo, D.; Bravo, J.; Rincón, A.; Lucas, M.M.; Pueyo, J.J. A nodule endophytic Bacillus megaterium strain isolated from Medicago polymorpha enhances growth, promotes nodulation by Ensifer medicae and alleviates salt stress in alfalfa plants. Ann. Appl. Biol. 2018, 172, 295–308.
- Nascimento, F.X.; Hernández, A.G.; Glick, B.R.; Rossi, M.J. Plant growth-promoting activities and genomic analysis of the stress-resistant Bacillus megaterium STB1, a bacterium of agricultural and biotechnological interest. Biotechnol. Rep. 2020, 25, e00406.
- Singh, R.P.; Jha, P.N. The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026.
- Li, H.; Lei, P.; Pang, X.; Li, S.; Xu, H.; Xu, Z.; Feng, X. Enhanced tolerance to salt stress in canola (Brassica napus L.) seedlings inoculated with the halotolerant Enterobacter cloacae HSNJ4. Appl. Soil Ecol. 2017, 119, 26–34.
- Bal, H.B.; Nayak, L.; Das, S.; Adhya, T.K. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 2013, 366, 93–105.
- Ilangumaran, G.; Schwinghamer, T.D.; Smith, D.L. Rhizobacteria from root nodules of an indigenous legume enhance salinity stress tolerance in soybean. Front. Sustain. Food. Syst. 2021, 4, 617978.
- Matse, D.T.; Huang, C.H.; Huang, Y.M.; Yen, M.Y. Effects of coinoculation of Rhizobium with plant growth promoting rhizobacteria on the nitrogen fixation and nutrient uptake of Trifolium repens in low phosphorus soil. J. Plant Nutr. 2020, 43, 739–752.
- Omara, A.E.; Hauka, F.; Afify, A.; Nour, E.M.; Kassem, M. The role of some PGPR strains to biocontrol Rhizoctonia solani in soybean and enhancement the growth dynamics and seed yield. Env. Biodivers. Soil Secur. 2017, 1, 47–59.
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2012, 14, 605–611.
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Plant growth-promoting rhizobacteria ameliorates salinity stress in pea (Pisum sativum). J. Plant Growth Regul. 2022, 41, 647–656.
- Jha, Y.; Subramanian, R.B. PGPR regulate caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol. Mol. Biol. Plants 2014, 20, 201–207.
- Hidri, R.; Mahmoud, O.M.-B.; Zorrig, W.; Mahmoudi, H.; Smaoui, A.; Abdelly, C.; Azcon, R.; Debez, A. Plant growth-promoting rhizobacteria alleviate high salinity impact on the halophyte Suaeda fruticosa by modulating antioxidant defense and soil biological activity. Front. Plant Sci. 2022, 13, 821475.
- Yun, P.; Xu, L.; Wang, S.S.; Shabala, L.; Shabala, S.; Zhang, W.Y. Piriformospora indica improves salinity stress tolerance in Zea mays L. plants by regulating Na+ and K+ loading in root and allocating K+ in shoot. Plant Growth Regul. 2018, 86, 323–331.
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272.
- del Amor, F.M.; Cuadra-Crespo, P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct. Plant Biol. 2011, 39, 82–90.
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.M.; Lee, I.J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ. Exp. Bot. 2017, 136, 68–77.
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768.
- Kabiraj, A.; Majhi, K.; Halder, U.; Let, M.; Bandopadhyay, R. Role of Plant Growth-Promoting Rhizobacteria (PGPR) for crop stress management. In Sustainable Agriculture in the Era of Climate Change; Springer: Cham, Switzerland, 2020; pp. 367–389.
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 104577.
- Li, X.; Sun, P.; Zhang, Y.; Jin, C.; Guan, C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 2020, 174, 104023.
- Habib, S.H.; Kausar, H.; Saud, H.M. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. Biomed Res. Int. 2016, 2016, 6284547.
- Kim, K.; Jang, Y.J.; Lee, S.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Alleviation of salt stress by Enterobacter sp. EJ01 in tomato and Arabidopsis is accompanied by up-regulation of conserved salinity responsive factors in plants. Mol. Cells 2014, 37, 109.
