Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Physiology
肾癌利用各种分子途径来响应和适应肾氧合的变化。特别是,缺氧诱导因子(HIF)(包括HIF-1,2,3)已被证明在肾脏疾病中被激活,并且在对缺氧的保护反应中起主要作用。HIF-1是一种异二聚体,由氧调节的HIF-1α亚基和组成型表达的HIF-1β亚基组成。在肾脏疾病中,HIF-1α的关键特征是保护性的,但它也具有负面影响,例如在肌肉减少症中。
- hypoxia-inducible factor-1α
- renal cancer
- renal ischemia/reperfusion injury
1. The Role of HIF-1α in RIRI
Currently, IR is the most widely used model for clinical AKI and renal transplant studies. Bioinformatic analysis of the GEO dataset and integration of gene expression profiles in a rat model of renal IRI identified HIF-1α signaling [15]. Mitochondrial dysfunction, induction of inflammation, apoptosis, autophagy, necroptosis and oxidative stress are the major factors in the pathogenesis of RIRI, and HIF-1α can protect against the kidney injury caused by RIRI through the above mechanisms (Figure 2).
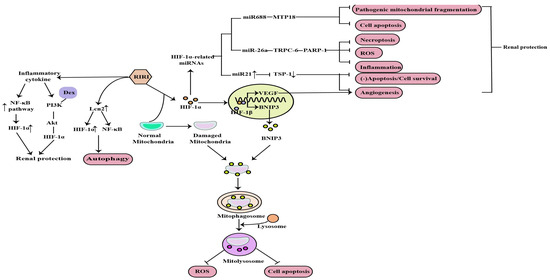
Figure 2. Mitochondrial dysfunction, induction of inflammation, apoptosis, autophagy, necroptosis, angiogenesis and ROS are the major factors in the pathogenesis of RIRI, and HIF−1α can protect against the kidney injury caused by RIRI through the above mechanisms (↑: elevated expression; ↓: decreased expression).
Apoptosis, autophagy and necroptosis are the important pathological mechanisms of RIRI. The NOD-like receptor thermal protein domain-associated protein 3(NLRP3) was reported to be involved in RIRI and NLRP3 knockout could protect against RIRI. Tetramethylpyrazine (TMP), a calcium antagonist with various pharmacological effects, has been widely used in the treatment of AKI. Sun et al. demonstrated that TMP can reduce NLRP3 protein expression in renal tissue and tubular cell apoptosis by reducing HIF-1α expression and improving renal function to alleviate RIRI [31]. Autophagy is activated by different types of stress, such as ischemia and inflammation, and is involved in AKI. Lipocalin 2 (Lcn2) is an important marker of renal injury and its production markedly increases in response to stimulation such as ischemia [32]. Mechanistically, Qiu et al. found that recombinant Lcn2 attenuated hypoxia-induced apoptosis and the downregulation of HIF-1α blunted Lcn2-induced autophagy and enhanced apoptosis. What’s more, Lcn2 attenuated NF-kB subunit p65 activation under hypoxia conditions. Thus, Lcn2 could protect against RIRI in mice through autophagy activation mediated by HIF-1α and NF-kB crosstalk [31]. Necroptosis is also a major contributor to the pathogenesis of ischemic AKI. Necrostatin-1 (Nec-1), an inhibitor of the kinase domain of receptor-interacting protein kinase-1 (RIP1), was previously reported to protect against RIRI. In previous studies, miR-26a has been identified as a potential novel target for the treatment RIRI. Moreover, previous bioinformatic studies have found that the transient receptor potential cation channel (TRPC6) is an upregulated and differentially expressed gene involved in the pathogenesis of I/R injury, and showed that it can prevent RIRI by inhibiting necroptosis. Shen et al. suggested that Nec-1 can effectively protect against RIRI by inhibiting necroptosis, oxidative stress, and inflammation, and may exert its effects through the mediation of the HIF-1α/miR-26a/TRPC6/PARP1 signaling pathway in vivo and vitro [33].
In addition, angiogenesis is one of the compensatory responses after RIRI. Promoting angiogenesis after reperfusion can prevent RIRI and improve the prognosis of AKI. HIF plays an important role in angiogenesis by upregulating vascular endothelial growth factor (VEGF) [34]. It has been reported that miR-21, induced by HIF, is essential in the process of vascular remodeling or angiogenesis in endothelial cells [35]. Thrombospondin 1 (TSP-1), is a multifunctional protein and was initially recognized as an endogenous inhibitor of angiogenesis. Xu et al. concluded that HIF-1α induced angiogenesis by upregulating not only vascular endothelial growth factor but also miR-21 via inhibiting a novel target gene TSP-1 contributing to the protective effect of HIF-1α on RIRI [36].
2. The Role of HIF-1α in Diabetic Nephropathy
DN is one of the most common models of CKD and the leading cause of ESRD. Earlier studies have demonstrated that hypoxia is an early event in the development and progression of experimental DN, and an increased HIF-1α expression in diabetic kidneys compared to the kidneys of control rats and normal human kidneys [37,38,39] (Figure 3). Ferroptosis is a recently discovered form of iron-dependent cell death. Heme is a main source of synthetic iron, and heme oxygenase (HO)-1 metabolizes heme into biliverdin/bilirubin, carbon monoxide, and ferrous iron. HO-1 can be induced by a variety of cues, including inflammatory mediators, oxidants, and physical or chemical stimuli, and HO-1 is one of the HIF target genes [40]. Chronic hypoxia due to renal ischemia induces the increase of HIF-1α in renal tubules of diabetic models, with the elevated HO-1 level [41,42]. Feng et al. indicated that ferroptosis might enhance DN and damage renal tubules in DN models through the HIF-1α/HO-1 pathway [43]. However, a study in 2020 demonstrated that HIF-1α improved mitochondrial dysfunction and restricted mitochondria-dependent apoptosis in the tubular cells of DN via the HO-1 pathway. In addition, the HIF-1α/HO-1 pathway is the pivotal pathway mediating tubular cell mitochondrial dynamics in DN [18].
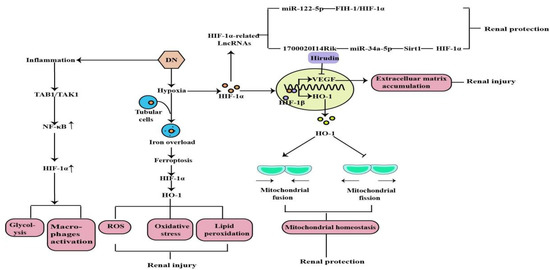
Figure 3. Mitochondrial dysfunction, lipid peroxidation, oxidative stress, ROS, machrophages activation, glycolysis, and the induction of inflammation are the major factors in the pathogenesis of DN, and HIF-1α can protect and aggravate the kidney injury caused by DN through the above mechanisms(↑: elevated expression).
Noncoding RNAs, arbitrarily separated into small ncRNAs and long noncoding RNAs (lncRNAs), are involved in multifarious physiological and pathological processes. A growing number of studies suggest that most genes are regulated by small ncRNAs, such as miRNA, and that miRNAs are involved in the pathogenesis of tubular injury in DN. The current study has demonstrated that miR-122-5p is up-regulated in the renal tubular cells of DN, and that it alleviates renal tubular cell death and kidney injury in DN. Factor inhibiting hypoxia-inducible factor-1 (FIH-1) was a direct target of miR-122-5p. Therefore, it indicated that miR-122-5p could ameliorate tubular injury in diabetic nephropathy via the FIH-1/HIF-1α pathway [44]. Numerous lncRNAs are reported to be involved in various kinds of diseases and oncogenesis, including DN [45]. By using RNA sequencing, we found many lncRNAs expressing differently in DN models, including the decreased expression level of lincRNAs-1700020I14Rik in DN in vitro and in vivo. In addition, increasing evidence shows that silent information regulator T1 (Sirt1) is involved in many important biological processes, including inflammation, renal interstitial fibrosis, autophagy under hypoxia condition, ageing, and oxidative stress. A decreased expression of Sirt1 was found in DN, and knockdown of Sirt1 expression may abolish the beneficial effects of the active component against renal damage in DN. Silencing Sirt1 could promote fibrosis factors and inflammation factors in DN glomerular measangial cells through promoting its downstream molecule HIF-1α expression. The results suggested that 1700020I14Rik plays an important regulatory role in DN through miR-34a-5p/Sirt1/HIF-1α signaling [46].
Hypoxia-induced inflammation plays a central role in the DN. Studies have shown that macrophage polarization occurs in renal inflammation and plays a decisive part in DN. Transforming growth factor β-activated kinase 1-binding protein 1 (TAB1) is a specific protein that interacts with transforming growth factor β-activated kinase 1 (TAK1). TAB1/TAK1 can activate NF-kB in bone marrow-derived macrophages (BMMs) of DN, thus upregulating HIF-1α activity and enhancing glycolytic metabolism. Zeng et al. concluded that the TAB1/NF-kB/HIF-1α signaling pathway regulates glycolysis and activation of macrophages in DN [47]. Previous studies have shown that high glucose-mediated tubulointerstitial accumulation of extracellular matrix (ECM) has an important role in the pathogenesis of DN. The HIF-1α/VEGF signaling pathway has previously been shown to be involved in the regulation of the ECM. Hirudin is an anticoagulant produced by the salivary glands of the medicinal leech. Hirudin reduces the deposition of ECM in DN models through the inhibition of the HIF-1α/VEGF pathway to protect kidney function and delay disease progression [48].
3. The Role of HIF-1α in Chronic Kidney Disease-Related Complications
3.1. Renal Anemia
Anemia is a common complication of CKD, mainly due to injured kidneys failing to produce sufficient amounts of EPO, which regulates red blood cell production. Hypoxia serves as the major stimulus of EPO production. Iron is essential in erythropoiesis. Iron deficiency also occurs in CKD patients due to the inadequate provision or absorption of dietary iron (Figure 4). HIF-1α is mainly induced in renal tubular and glomerular epithelial cells and in papillary interstitial cells, whereas HIF-2α is expressed in endothelial cells and fibroblasts upon hypoxic stimulation, suggesting that HIF-2α is the main regulator of EPO production [49]. Under hypoxic conditions, HIF-2α regulates EPO expression in combination with hypoxia response elements on the EPO gene in the kidney and liver [50]. Voit et al. revealed that EPO production in the kidney can also be regulated by HIF-1α, which is degraded under normoxic conditions by HIF-prolyl hydroxylase (HIF-PHD) [19]. The discovery of prolyl hydroxylase domain (PHD) enzymes as regulators of hypoxia-inducible factor (HIF)-dependent erythropoiesis has led to the development of novel therapeutic agents for renal anemia [51]. Roxadustat, the first small-molecule PHI, can lead to increased EPO production, better iron absorption, and amelioration of anemia in CKD [19]. In addition, HIF-2α also regulates iron metabolism by stimulating duodenal cytochrome B (DCYTB) and divalent metal transporter-1 (DMT1) expression.
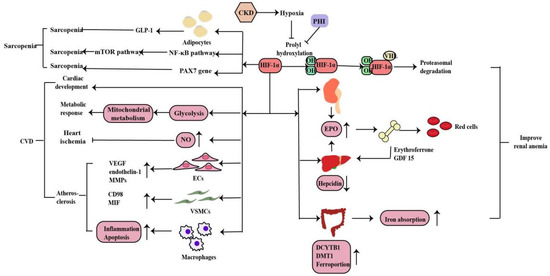
Figure 4. HIF-1α regulates EPO gene in the kidney and liver and also regulates iron metabolism by stimulating DCYTB and DMT1 expression. Inhibition of HIF-PHD leads to increased EPO production, better iron absorption, and amelioration of anemia in CKD. Under hypoxia, HIF signaling affects the cardiac development, metabolic response, heart ischemia and atherosclerosis to CVD in myriad ways. And HIF-1α plays a dual role in sarcopenia through the mechanisms described above (↑: elevated expression; ↓: decreased expression).
3.2. Cardiovascular Disease
CVD is the main cause of death in patients with CKD. Hypoxia is also a promoting factor of myocardial infarction, cardiac remodeling, atherosclerosis and peripheral arterial disease in CKD patients. Under hypoxia, HIF signaling affects the development, metabolic response, ischemia and atherosclerosis in heart disease in myriad ways (Figure 4). Animal experiments have shown that the HIF-1α deletion has a wide range of cardiac abnormalities, including septation and trabeculation, and is affected by specific alleles and the mouse genetic background [52]. Thus, HIF-regulated transcription is necessary for important cardiac developmental events. Cellular metabolism is inextricably linked with cardiac contractility and HIF plays a crucial role in shaping the metabolic response in the heart. HIF-1α promotes glycolysis during hypoxia and influences mitochondrial metabolism to proper cardiometabolic function [20]. As a vasodilator, NO plays a vital role in the regulation of vascular tone through the regulating cGMP in smooth muscle cells, S-nitrosylation of target proteins, the activation of sarco/endoplasmic reticulum calcium ATPase and the production of cyclic inosine monophosphate. HIF-1α can activate inducible nitric oxide synthase (iNOS) gene expression by increasing NO synthesis to limit ischemic damage in the heart [53]. The effects of HIF-1α in the three most important cell types of atherosclerosis--macrophages, vascular smooth muscle cells (VSMCs), and endothelial cells (ECs) are essential. Whereas HIF-1α directly induces VEGF, endothelin-1, and matrix metalloproteinases (MMPs) in endothelial cells to facilitate angiogenesis, its effect on vascular smooth muscle cells is to induce proliferation of these cells in the atheroma by up-regulating factors such as CD98 and macrophage migration inhibitory factor (MIF). HIF-1α also modulates the function of diseased macrophage foam cells by making the cells more inflammatory and apoptotic while simultaneously inhibiting their ability to metabolize lipids [20].
3.3. Sarcopenia
肌肉减少症是CKD患者的并发症之一。缺氧会导致肌肉氧输送减少,这在 CKD 贫血中更为明显。从机制上讲,HIF-1α缺失刺激人脂肪细胞中GLP-1的分泌,从而导致缺氧时的肌肉减少[54]。此外,缺氧通过激活HIF-1α和NF-kB分解代谢途径并抑制雷帕霉素(mTOR)途径的合成代谢哺乳动物靶标来诱导肌肉萎缩[55,56]。Cirillo等人发现HIF-1α及其靶基因在肌肉减少症以及卫星细胞的主要干细胞标志物PAX7中的表达减少,而萎缩标志物MURF1增加。此外,HIF-1α及其靶基因的药理学激活导致骨骼肌萎缩减少和PAX7基因表达激活。因此,HIF-1α在肌肉减少症中起作用,并参与卫星细胞稳态,但需要进一步研究[57](图4)。
4. HIF-1α在肾癌中的作用
缺氧在很多类型的实体瘤中很常见,其中肿瘤细胞迅速增殖并形成大的实体瘤肿块,导致这些肿块周围的血管阻塞和压迫。这些异常血管通常不能正常工作,导致2向中心肿瘤区域供应[58]。肾癌是一种常见的泌尿系统恶性肿瘤,转移性疾病的治疗选择有限。大多数透明细胞肾细胞癌(ccRCC)与von Hippel-Lindau肿瘤抑制因子(pVHL)功能的丧失和缺氧途径的失调有关。VHL肿瘤抑制因子在大多数ccRCC肿瘤中失活。VHL 是含有延长蛋白 B 和 C、Cullin-2 和 Rbx1 的 E3 泛素连接酶复合物的底物识别组分,其靶向 HIF 的羟基化、氧敏感α亚基,用于泛素化和 26S 蛋白酶体降解。VHL丢失导致HIF靶标的组成性激活,包括促血管生成因子VEGF和血小板衍生生长因子(PDGF)[59]。活化的HIF-1通过转录激活100多个下游基因,调节肿瘤存活和进展所需的重要生物过程,在肿瘤细胞对氧变化的适应性反应中起着至关重要的作用。对HIF的进一步研究将有助于开发癌症疗法[60,61](图5)。
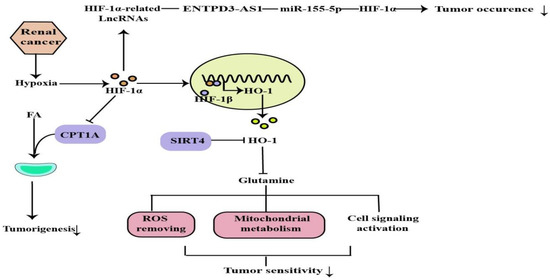
图5.ROS去除、线粒体代谢、细胞信号激活受HIF-1α调控,可通过上述机制调节肿瘤发生和敏感性(↓:表达降低)。
This entry is adapted from the peer-reviewed paper 10.3390/molecules27217318
This entry is offline, you can click here to edit this entry!
