The aorta is the largest artery in the body, delivering oxygenated blood from the left ventricle to all organs. Dissection of the aorta is a lethal condition caused by a tear in the intimal layer of the aorta, followed by blood loss within the aortic wall and separation of the layers to full dissection. The aorta can be affected by a wide range of causes including acute conditions such as trauma and mechanical damage; and genetic conditions such as arterial hypertension, dyslipidaemia, and connective tissue disorders; all increasing the risk of dissection. Both rapid diagnostic recognition and advanced multidisciplinary treatment are critical in managing aortic dissection patients.
- aortic dissection
- diagnosis
- TEVAR
- prognosis
1. Introduction

2. Epidemiology
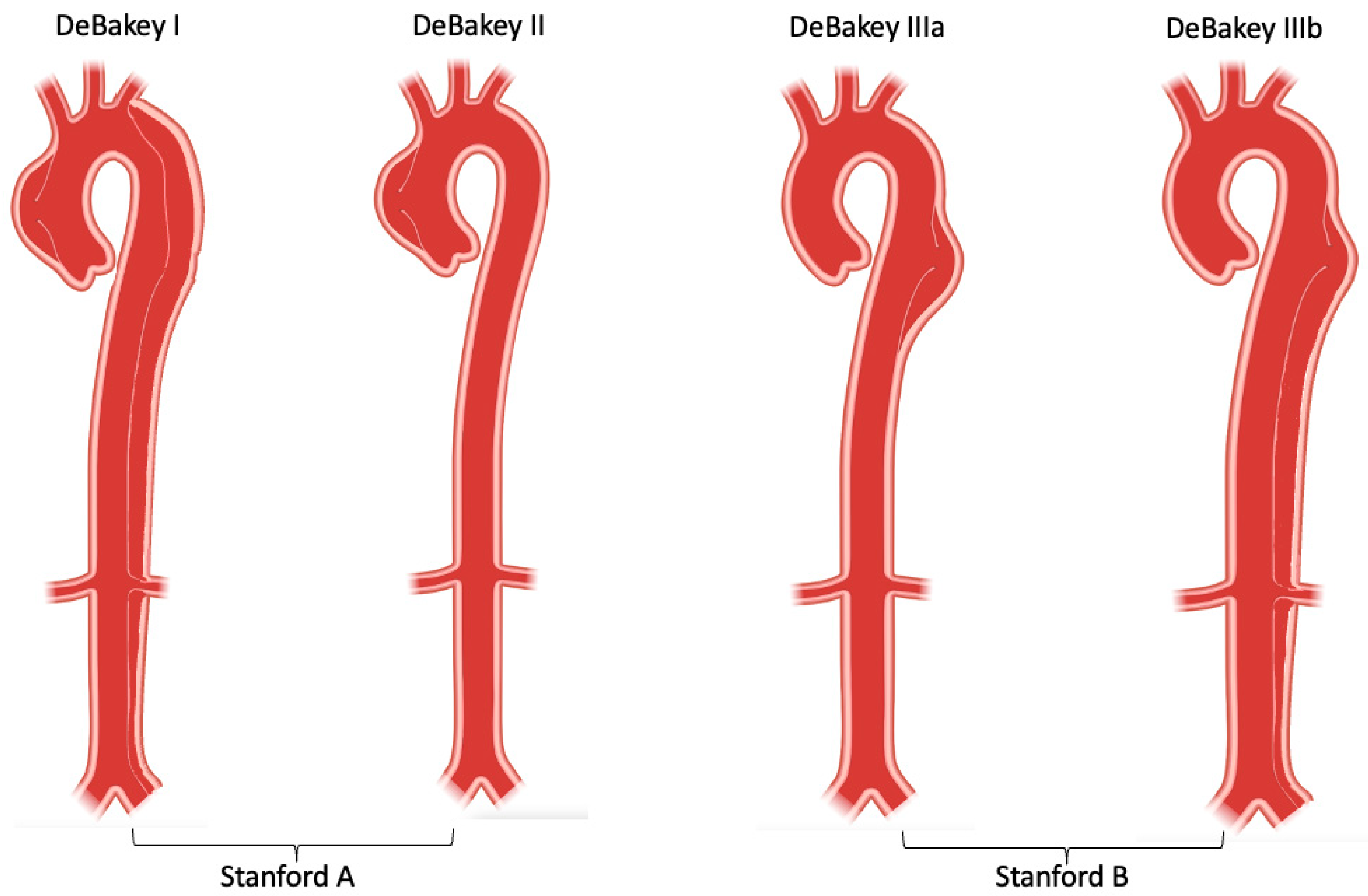
3. Classification
This entry is adapted from the peer-reviewed paper 10.3390/life12101606
References
- Nienaber, C.A.; Clough, R.E. Management of acute aortic dissection. Lancet 2015, 385, 800–811.
- Nienaber, C.A.; Clough, R.E.; Sakalihasan, N.; Suzuki, T.; Gibbs, R.; Mussa, F.; Jenkins, M.P.; Thompson, M.M.; Evangelista, A.; Yeh, J.S.; et al. Aortic dissection. Nat. Rev. Dis. Prim. 2016, 2, 16071.
- Daily, P.O.; Trueblood, H.W.; Stinson, E.B.; Wuerflein, R.D.; Shumway, N.E. Management of acute aortic dissections. Ann. Thorac. Surg. 1970, 10, 237–247.
- Debakey, M.E.; Henly, W.S.; Cooley, D.A.; Morris, G.C., Jr.; Crawford, E.S.; Beall, A.C., Jr. Surgical Management of Dissecting Aneurysms of the Aorta. J. Thorac. Cardiovasc. Surg. 1965, 49, 130–149.
- Clouse, W.D.; Hallett, J.W., Jr.; Schaff, H.V.; Spittell, P.C.; Rowland, C.M.; Ilstrup, D.M.; Melton, L.J., 3rd. Acute aortic dissection: Population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin. Proc. 2004, 79, 176–180.
- Meszaros, I.; Morocz, J.; Szlavi, J.; Schmidt, J.; Tornoci, L.; Nagy, L.; Szep, L. Epidemiology and clinicopathology of aortic dissection. Chest 2000, 117, 1271–1278.
- Howard, D.P.; Banerjee, A.; Fairhead, J.F.; Perkins, J.; Silver, L.E.; Rothwell, P.M.; Oxford Vascular, S. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013, 127, 2031–2037.
- Smedberg, C.; Steuer, J.; Leander, K.; Hultgren, R. Sex differences and temporal trends in aortic dissection: A population-based study of incidence, treatment strategies, and outcome in Swedish patients during 15 years. Eur. Heart J. 2020, 41, 2430–2438.
- Howard, D.P.; Sideso, E.; Handa, A.; Rothwell, P.M. Incidence, risk factors, outcome and projected future burden of acute aortic dissection. Ann. Cardiothorac. Surg. 2014, 3, 278–284.
- Evangelista, A.; Isselbacher, E.M.; Bossone, E.; Gleason, T.G.; Eusanio, M.D.; Sechtem, U.; Ehrlich, M.P.; Trimarchi, S.; Braverman, A.C.; Myrmel, T.; et al. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation 2018, 137, 1846–1860.
- Pape, L.A.; Awais, M.; Woznicki, E.M.; Suzuki, T.; Trimarchi, S.; Evangelista, A.; Myrmel, T.; Larsen, M.; Harris, K.M.; Greason, K.; et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J. Am. Coll. Cardiol. 2015, 66, 350–358.
- Olsson, C.; Thelin, S.; Stahle, E.; Ekbom, A.; Granath, F. Thoracic aortic aneurysm and dissection: Increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006, 114, 2611–2618.
- DeMartino, R.R.; Sen, I.; Huang, Y.; Bower, T.C.; Oderich, G.S.; Pochettino, A.; Greason, K.; Kalra, M.; Johnstone, J.; Shuja, F.; et al. Population-Based Assessment of the Incidence of Aortic Dissection, Intramural Hematoma, and Penetrating Ulcer, and Its Associated Mortality From 1995 to 2015. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004689.
- Melvinsdottir, I.H.; Lund, S.H.; Agnarsson, B.A.; Sigvaldason, K.; Gudbjartsson, T.; Geirsson, A. The incidence and mortality of acute thoracic aortic dissection: Results from a whole nation study. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2016, 50, 1111–1117.
- McClure, R.S.; Brogly, S.B.; Lajkosz, K.; Payne, D.; Hall, S.F.; Johnson, A.P. Epidemiology and management of thoracic aortic dissections and thoracic aortic aneurysms in Ontario, Canada: A population-based study. J. Thorac. Cardiovasc. Surg. 2018, 155, 2254–2264.e4.
- Huckaby, L.V.; Sultan, I.; Trimarchi, S.; Leshnower, B.; Chen, E.P.; Brinster, D.R.; Myrmel, T.; Estrera, A.L.; Montgomery, D.G.; Korach, A.; et al. Sex-Based Aortic Dissection Outcomes From the International Registry of Acute Aortic Dissection. Ann. Thorac. Surg. 2022, 113, 498–505.
- Yuan, X.; Mitsis, A.; Ghonem, M.; Iakovakis, I.; Nienaber, C.A. Conservative management versus endovascular or open surgery in the spectrum of type B aortic dissection. J. Vis. Surg. 2018, 4, 59.
- Rylski, B.; Perez, M.; Beyersdorf, F.; Reser, D.; Kari, F.A.; Siepe, M.; Czerny, M. Acute non-A non-B aortic dissection: Incidence, treatment and outcome. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2017, 52, 1111–1117.
- Sievers, H.H.; Rylski, B.; Czerny, M.; Baier, A.L.M.; Kreibich, M.; Siepe, M.; Beyersdorf, F. Aortic dissection reconsidered: Type, entry site, malperfusion classification adding clarity and enabling outcome prediction. Interact Cardiovasc. Thorac. Surg. 2020, 30, 451–457.
- Mitsis, A.; Yuan, X.; Cambronero-cortinas, E.; Nienaber, C.A. New developments in the treatment of type B aortic dissection. Ital. J. Vascualr Endovasc. Surg. 2017, 24, 118–126.
- Kato, N.; Shimono, T.; Hirano, T.; Suzuki, T.; Ishida, M.; Sakuma, H.; Yada, I.; Takeda, K. Midterm results of stent-graft repair of acute and chronic aortic dissection with descending tear: The complication-specific approach. J. Thorac. Cardiovasc. Surg. 2002, 124, 306–312.
- Bogdan, Y.; Hines, G.L. Management of acute complicated and uncomplicated type B dissection of the aorta: Focus on endovascular stent grafting. Cardiol. Rev. 2010, 18, 234–239.
- Acosta, S.; Blomstrand, D.; Gottsater, A. Epidemiology and long-term prognostic factors in acute type B aortic dissection. Ann. Vasc. Surg. 2007, 21, 415–422.
- Nienaber, C.A.; Kische, S.; Rousseau, H.; Eggebrecht, H.; Rehders, T.C.; Kundt, G.; Glass, A.; Scheinert, D.; Czerny, M.; Kleinfeldt, T.; et al. Endovascular repair of type B aortic dissection: Long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ. Cardiovasc. Interv. 2013, 6, 407–416.
- Brunkwall, J.; Kasprzak, P.; Verhoeven, E.; Heijmen, R.; Taylor, P.; Trialists, A.; Alric, P.; Canaud, L.; Janotta, M.; Raithel, D.; et al. Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic remodelling: 1 year results of the ADSORB trial. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 285–291.
- Kamman, A.V.; Brunkwall, J.; Verhoeven, E.L.; Heijmen, R.H.; Trimarchi, S.; Kasprzak, P.; Alric, P.; Schumacher, H.; Fabiani, J.N.; Eckstein, H.-H.; et al. Predictors of aortic growth in uncomplicated type B aortic dissection from the Acute Dissection Stent Grafting or Best Medical Treatment (ADSORB) database. J. Vasc. Surg. 2017, 65, 964–971.e3.
- Matsushita, A.; Tabata, M.; Mihara, W.; Shimamoto, T.; Komiya, T.; Takanashi, S.; Tobaru, T.; Nakao, T.; Nakamura, S.; Sato, Y. Risk score system for late aortic events in patients with uncomplicated type B aortic dissection. J. Thorac. Cardiovasc. Surg. 2020, 159, 2173–2183.e1.
- Sailer, A.M.; van Kuijk, S.M.; Nelemans, P.J.; Chin, A.S.; Kino, A.; Huininga, M.; Schmidt, J.; Mistelbauer, G.; Baumler, K.; Chiu, P.; et al. Computed Tomography Imaging Features in Acute Uncomplicated Stanford Type-B Aortic Dissection Predict Late Adverse Events. Circ. Cardiovasc. Imaging 2017, 10, e005709.
- Ante, M.; Mylonas, S.; Skrypnik, D.; Bischoff, M.S.; Rengier, F.; Brunkwall, J.; Bockler, D. Prevalence of the Computed Tomographic Morphological DISSECT Predictors in Uncomplicated Stanford Type B Aortic Dissection. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 525–533.
- DeBakey, M.E.; Beall, A.C., Jr.; Cooley, D.A.; Crawford, E.S.; Morris, G.C., Jr.; Garrett, H.E.; Howell, J.F. Dissecting aneurysms of the aorta. Surg. Clin. North Am. 1966, 46, 1045–1055.
- Booher, A.M.; Isselbacher, E.M.; Nienaber, C.A.; Trimarchi, S.; Evangelista, A.; Montgomery, D.G.; Froehlich, J.B.; Ehrlich, M.P.; Oh, J.K.; Januzzi, J.L.; et al. The IRAD classification system for characterizing survival after aortic dissection. Am. J. Med. 2013, 126, 730.e19–730.e24.
