Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pediatrics
Oxidative stress is a condition determined by an imbalance between antioxidant and oxidative factors. Oxidative stress can have serious consequences on our organism. Indeed, it causes both necrosis and cell apoptosis, determining cellular aging, increased carcinogenesis, vascular stiffening, increased autoimmune diseases, and muscle decay.
- oxidative stress
- antioxidant
- pediatrics
- FASD
1. Introduction
Oxidative stress is a condition in which the balance between antioxidant and proxying factors is altered in favor of the production of oxidizing species; this determines the overproduction of radical oxygen species, defined as ROS, and can be associated with a reduction in the synthesis of antioxidant factors, as shown in Figure 1 [1,2]. In the correct balance of the cellular oxidative state, ROS are necessary for the performance of some functions [3]. In fact, among the functions performed by ROS, there are the degradation of pathogens, the regulation of cardiac and vascular activities, the regulation of intracellular calcium concentration, and the phosphorylation or the dephosphorylation of proteins [3,4]. In the context of pediatric syndromes, the role of oxidative stress is not yet fully elucidated, and there is no review of literature that compares the various types of oxidative stress in different syndromes [5].
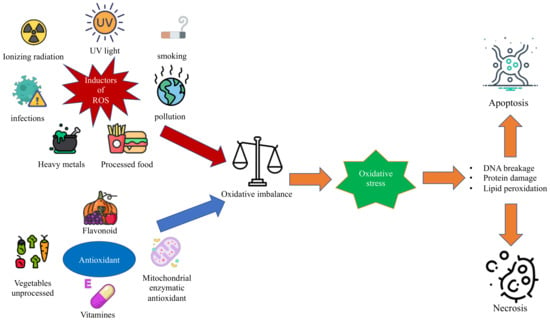
Figure 1. The figure shows the oxidative imbalance between ROS and antioxidant factors that leads to oxidative stress and the related consequences on the cell.
2. Pediatrics Syndromes Associated with Oxidative Stress
2.1. Fetal Alcohol Spectrum Disorders
FASD is the term adopted to identify the group of symptoms affecting cognitive and behavioral functioning that can be found in newborns whose mothers consumed alcohol during pregnancy [80,81,82]. It may be interesting to stress that, even if the latest Diagnostic and Statistical Manual of Mental Disorders (DSM-V) does not consider FASD to be a specific clinical mental health disorder, it does acknowledge the need for further studies on neurobehavioral disorders associated with prenatal alcohol exposure [83,84].
FASD is a disease with no genetic origin but is due solely and exclusively to the mother’s consumption of alcohol during pregnancy [85]. At present, a minimum dose determining the disease has not yet been identified. For this reason, it is absolutely forbidden to consume alcohol during pregnancy, even in minimal quantities [86].
The effect of alcohol consumption can vary extremely [87], ranging from mild intellectual and/or behavioral impairments to fetal alcohol syndrome (FAS), whose prevention has become the main goal of public health workers since the identification of the damages alcohol consumption can provoke to the fetus [88]. For instance, it should be noted that FAS has been classified as the leading cause of mental retardation worldwide and that, among neurobehavioral and developmental abnormalities, FAS would benefit the most from prevention—that is to say, the foremost preventable cause [84,89,90,91,92,93,94,95].
An interesting aspect of the matter is comparing the causal link between oxidation and FASD as opposed to other genetic disorders. In fact, the patients with FASD are conceived as perfectly healthy, and the oxidation provoked by alcohol is the cause of the pathology since it produces its effect on an otherwise physiological substrate [84,96,97].
More specifically, the scientific community has noticed a strong effect of alcohol exposure on the hippocampal proteome, culminating with the alternation of more than 600 hippocampal proteins playing important roles in the axonal growth regulation, such as annexin A2, nucleobindin-1, and glypican-4, regulators of cellular growth and developmental morphogenesis and, in the cerebellum, cadherin-13, reticulocalbin-2, and ankyrin-2 [98].
The ethanol contained in alcoholic beverages is first converted into acetaldehyde by various enzymes such as alcohol dehydrogenase (ADH), cytochrome CYP2E1, and catalase [99,100]. In the brain, the two most important pathways seem to be those mediated by cytochrome CYP2E1 and catalase, which metabolize 20% and 60% of ethanol, respectively [100]. On the other hand, the most represented pathway for the liver and stomach is mediated by alcohol dehydrogenase, which metabolizes 90% of the ethanol taken [101].
The acetaldehyde produced is then converted into acetate by acetaldehyde dehydrogenase (ALDH), which in turn is converted into acetyl-coenzyme-A in the liver [102].
A major problem of alcohol intake during pregnancy is related to the fact that the fetus has limited or null abilities in the metabolization and elimination of alcohol. Indeed, the various enzymes involved gradually increase their activity during the various stages of gestation [84]. For example, cytochrome CYP2E1 increases its activity compared to an adult, from 40% in the second semester to 80% in the third semester [84]. Therefore, the fetus is likely to be able to metabolize less than half of the alcohol taken by the mother [100].
The increase in ROS in FASD also appears to be due to NOX enzymes belonging to the NADPH-dependent family of enzymes [103]. The NOXs enzymes are expressed at the level of microglia, astrocytes, and the vascular system at the cerebral level, with an important role in the appropriate brain development [104]. The isoforms most involved in ROS production are NOX2 and NOX4 [100]. In FASD patients, it would appear that early exposure to ethanol during pregnancy would increase the activity of NOX isoforms with a significant increase in ROS, cell damage, and ultimately apoptosis [100]. This pathway, in conjunction with that related to the reduced fetal detoxification activity of CYP2E1, would seem to explain the drastic increase in ROS and the consequent phenotype of FASD patients [105].
Even if the mechanisms of the alcohol-induced neuropathology in regions of high vulnerability remain to be comprehensively determined, the teratogenic effects are thought to be the ultimate result of the ethanol-induced dysregulation of a variety of intracellular pathways, which ultimately culminate in toxicity and cell death [85]. The generation of ROS as the possible result of ethanol exposure produces an imbalance in the intracellular redox state, leading to an overall increase in oxidative stress [106]. This would explain the predominant effect that alcohol has on the brain regarding neurobehavioral impairment and deficient brain growth since brain tissue is rich in fatty acids, which chemically are the perfect substrate for the ROS [86]. The most valid biochemical explication would be that the enzyme CYP2E1, whose presence in the brain overlaps with its organogenesis, oxidizes ethanol, generating a hydroxyethyl or superoxide radical, which would target polyunsaturated fatty acid side chains in brain tissue membranes [98,105].
As a consequence, fetal brain tissue results in damage during organogenesis, manifesting neurological dysfunctions after birth [86,104,105,107].
2.2. Williams-Beuren Syndrome
Williams-Beuren Syndrome (WBS) is a rare genetic disease with multisystemic involvement, affecting nearly 1 out of 7500–10,000 people. A deletion of a group of genes, situated on chromosome 7 (7q11.23), including the ELN (elastin; OMIM *130,160) gene, is the WBS cause. These genes encode elastin, whose deletion is responsible for the cardiovascular traits and accelerated aging in patients with this disease [113,114]. The common features of this syndrome are represented by the following: facial dysmorphisms, cardiovascular malformations, endocrinological alterations, and intellectual and cognitive disturbances [113]. WBS individuals often show signs of mildly accelerated aging such as cataracts, graying of hair during adolescence, high-frequency sensorineural hearing loss, senile emphysema, premature wrinkling of the skin, and a precipitous age-associated decrease in episodic memory [114,115,116]. The oxidative stress role in these patients is less known than in other genetic disorders; nevertheless, recent studies have shown a correlation between the elastin-insufficiency of WBS and cardiovascular and respiratory diseases [117,118]. For the cardiovascular system, the major impact of ROS seems to be in the hypertension predisposition, in fact as stated before the ELN is a crucial component of the vascular wall providing recoil to elastic vessels [117,118].
Arteries with decreased ELN content are less compliant and develop structural modifications that include elevated numbers of smooth muscle and elastic lamellae; consequently, people with ELN deficiency have developmentally, rather than environmentally, elicited vascular stiffness [119]. They also show anatomical differences in branching and arterial tortuosity that lead to a turbulent flow and increased hemodynamic stress on vessel walls [119]. Recently, increasing pieces of evidence demonstrate that ROS production, particularly O2•− and H2O2, through activation of vascular NADPH oxidases, has a central role in vascular mechanotransduction [120,121]. Smooth and endothelial muscle cells express different NADPH oxidases consisting of multiple oxidases and regulatory subunits [122,123]. Other lines of evidence suggest also that hemodynamic forces can either directly or indirectly activate vascular NADPH oxidase-derived ROS production [122,123]. Abundant findings suggest that hypertension might be associated with the potentiated activity of the vascular NADPH oxidases type 1 and 2 having a regulatory subunit, the defined p47phox. This molecule is encoded by the NCF1 (neutrophil cytosolic factor 1) gene located on the telomeric region of chromosome 7 [118]. The lower expression of NCF1 is related to lower blood pressure and minor ROS production, so if the deletion on chromosome 7 extends to include the NCF1 gene, the incidence of hypertension decreases [118,124].
In the respiratory system, WBS subjects with ELN haploinsufficiency may be predisposed to the early development of pulmonary emphysema, the elastin in fact, is a key component of elastic fibers within the lung [124]. Emphysema, a subtype of chronic obstructive pulmonary disease (COPD), is characterized by progressive destruction and loss of elastic fibers, but this is not the only possible mechanism [116]. In WBS, there is a mitochondrial dysfunction. In fact, in the primary fibroblasts of patients affected, decreased basal respiration and maximal respiratory capacity were found, as well as increased ROS generation and decreased ATP synthesis [117]. This mitochondrial dysfunction may be due to the loss of DNAJC30, a gene included in the WBS critical region (WBSCR) [125]. Recent studies have uncovered significant mitochondrial signatures in chronic lung diseases, perturbations of cellular homeostatic programs associated with mitochondrial dysfunction in chronic lung diseases include modulation of the cellular autophagy program, its mitochondria-specific subtype (mitophagy), and associated changes in mitochondrial dynamics and activation of cell death pathways such as apoptosis and necrosis [102,126,127,128]. In addition, mitochondrial dysfunction may have differential and cell type-specific functional consequences in different lung cell types (e.g., epithelial cells, fibroblasts, immune cells), which may differentially impact disease progression, leading to divergent outcomes such as the development of fibrosis or emphysema [129].
Mitochondrial dysfunction could also be related to impaired brain development. Removal of DNAJC30 in mice resulted in hypofunctional mitochondria, diminished morphological features of neocortical pyramidal neurons, and altered behaviors reminiscent of WBS [5]. The mitochondrial features are consistent with our observations of decreased integrity of oxidative phosphorylation supercomplexes and ATP-synthase dimers in WBS [130]. Thus, we identify DNAJC30 as an auxiliary component of ATP-synthase machinery and reveal mitochondrial maladies as underlying certain defects in brain development and function associated with WBS [125].
2.3. Ataxia-Telangiectasia
Ataxia-telangiectasia (A-T) is an autosomal recessive disease eliciting several pathologies in the first two decades of life, including immunodeficiency, insulin resistance, telangiectasias, cerebellar ataxia, T-lymphoid tumors, and radiosensitivity [5,134]. Among these, T-cell malignancies and cerebellar ataxia are the most incapacitating phenotypes of this disease [5]. A-T is due to mutations in the Ataxia Telangiectasia Mutated (ATM) gene [135]. The gene encodes a serine/threonine protein kinase belonging to the phosphoinositide 3-kinase (PI3K)-related protein kinase family [135]. ATM plays a main role at the beginning of cellular responses to DNA double-strand breaks [136,137]. Nevertheless, some of the phenotypic disruptions observed in A-T individuals are not easily elucidated only by changes in DNA damage response (DDR) paths [136,137]. Since the antioxidant treatment of ATM-null mice improves intrinsic defects in stem cell renewal and might contribute to the delay of their tumor onset, it has been hypothesized that augmented accumulation of intracellular ROS, associated with ATM impairments, may contribute to these diseases [138,139].
The first possible mechanism described is mitochondrial dysfunction. In fact, the ATMs role ATM in maintaining mitochondrial functionality is well shown [102,140,141]. The ATM loss in vivo produces mitochondria disruptions, causing overproduction of ROS, a marked reduction in ATP, and ultrastructural abnormalities [139]. Furthermore, the selective exclusion of impaired mitochondria, known as mitophagy, is strongly impaired, leading to dysfunctional organelles accumulation [5]. The following other pieces of evidence have been reviewed also in neuroblastoma: the depletion of ATM produces comparable mitochondrial phenotypes and mitophagy changes [141]. Studies on thymocytes isolated from mutated mice showed mitochondrial abnormalities in ATM-deficient thymic cells, with disorganized structure and swollen appearance as well as a complex I activity significantly decreased that led to an abnormal generation of ROS taken together, demonstrating the relation between ATM deficiency and mitochondrial dysfunction [5,140].
Noteworthy A-T findings on alternative ROS sources were presented by Weyemi et al., who demonstrated that NADPH oxidase 4 (NOX4) in A-T cells could be a key instrument of oxidative stress [142]. Indeed, it was shown that NOX4, which constitutively triggers ROS in a variety of tissues and cell types as well as has a subtle role in oxidative DNA damage and the consequent senescence, was quite up-regulated in A-T individuals, comparable to normal cells with an ATM protein kinase privation [28,143]. Additionally, NOX4 is correlated with higher oxidative damage and apoptosis [6,142]. Moreover, NOX4 inactivation decreased cancer incidence (lymphoma) in ATM-deficient mice compared to control mice [6,142].
Semlitsch et al. demonstrated the following other important sources of damage in A-T patients: a specific oxidation product: the ox-LDL (oxidized low-density lipoprotein) [144,145]. OxLDL is a potent proinflammatory chemoattractant for macrophages and T-lymphocytes. It is also cytotoxic for endothelial cells and stimulates them to release soluble inflammatory molecules [146]. In addition, oxLDL has turned out to be highly immunogenic and promotes changes in cell cycle protein expression and subsequent translocation and activation of transcription factors [145,147]. These events help to perpetuate a cycle of vascular inflammation and lipid/protein dysregulation within the artery wall and also may create a cellular prothrombotic state that complicates later stages of atherosclerosis [147].
OxLDL generates oxidative stress in the vascular system induced phosphorylation of ATM and downstream activation of p21 in fibroblasts and endothelial cells. ATM-deficient cells are extremely sensitive to the toxic effects of ROS, especially H2O2 and nitric oxide developing an increased DNA fragmentation [148,149].
In conclusion, oxidative stress in A-T is related to various sources such as mitochondrial dysfunction, augmented production of ROS by the up-regulation of NOX4, and increased sensibility to ox-LDL with DNA fragmentation [6,145].
2.4. Down Syndrome
Down syndrome (DS) is a congenital disorder caused by a complete or partial trisomy of Chr21 (HSA21), and it is the most common genetic cause of significant intellectual disability, with an incidence of around 1:800 births [152]. Most DS cases (95%) are caused by non-disjunction of chromosomes in meiosis I during the formation of gametes, while 3.2% are caused by translocation, and 1.8% of residual DS cases are caused by mosaicism [153]. The effects of trisomy 21 can be very different from one individual to the next, and not every DS subject shows the same phenotypic features. The main alteration is represented by intellectual disability, other common features are congenital heart disease, Alzheimer’s disease, leukemia, hypotonia, motor disorders, and various physical anomalies [154]. Although pathological mechanisms leading to DS phenotypes are still uncertain, it is evident that the presence of the third chromosome 21 is responsible for altered development during embryogenesis and organogenesis [155]. Many of its clinical features were also studied as possible consequences of oxidative stress and cellular senescence since the change in chromosome 21 affects genes playing key roles in the redox state regulation [117,156]. When associated with other redox imbalance genetic diseases, DS has been broadly investigated [157,158]. Several findings showed that changes in proteins and genes involved in ATP consumption, mitochondrial pathways, and increased ROS production may explain the wide variety of phenotypes [159].
The most important genes that are involved in the increase in oxidative stress levels found in DS individuals and in the Ts65Dn mouse model are SOD1, APP, BACH1, Et2, S100B, and CRB [160].
The first chromosome 21 gene that was characterized and identified in different DS tissues was SOD-1 (Cu/Zn superoxide dismutase 1) (OMIM *147,450) [161]. It acts as an antioxidant defense that catalyzes the dismutation of superoxide radicals (O2−) to hydrogen peroxide (H2O2), then metabolized to water by catalases (CAT) and glutathione peroxidase (GTPx) [162]. SOD-1 was shown to be approximately 50% higher than normal in a wide range of DS tissues and cells, including B and T lymphocytes, fibroblasts, and erythrocytes [163]. Furthermore, SOD-1 overexpression in the brain was not associated with a parallel CAT and GTPx elevation, determining an imbalance in the ratio of SOD-1 to CAT and GTPx levels, leading to an H2O2 accumulation and consequent damage [164]. Surprisingly, DS tissues, including the brain, show changes in the SOD-1/GTPx activity ratio [165]. Associated with CAT and GTPx, a decreased expression of peroxiredoxin 2 was also found in the DS fetal brain, which contributes to the improved susceptibility of DS neurons to undergoing oxidative damage [166]. Since SOD-1 may play an important role in the pathogenesis of Ts21, it could be used as a potential biomarker for the prenatal diagnosis of Ts21 [167].
In addition to the well-recognized role of SOD-1, alterations in the oxidative imbalance could also be caused by the over-production of beta-amyloid (Aβ), due to the triplication of APP. APP (Amyloid Beta A4 Precursor Protein) (OMIM *104,760) encodes for a precursor protein of Aβ. Its overexpression leads to Aβ deposition and increases the formation of senile plaques, a main neuropathological finding of Alzheimer’s disease [154]. DS is the most common cause of early-onset Alzheimer’s disease-dementia [168]. In patients with DS, the increase in APP expression is strongly associated with Aβ deposition in adult life and the early and increased formation of senile plaques [158]. Oxidative stress and early plaque formation in the brain are closely connected. In fact, ROS damage increases the probability of the formation of protein aggregates as it obstructs the normal processes of protein elimination. The Aβ aggregates can be targets of oxidative processes, inserted as oligomers within the cell membrane and promoting a process of lipid peroxidation (LPO) [117].
BACH1 (BTB domain and CNC homolog 1; OMIM *602,751), encoded on Hsa21, is another key element in the regulation of the antioxidant response in DS [159]. It is a transcription repressor that inhibits selected gene transcription involved in stress response, such as heme oxygenase-1 (HO-1) and NADPH. The BACH1 overexpression potentiates ROS production from the endothelial cell’s mitochondria [159]. DS mouse model studies and investigations into DS patients demonstrated that BACH1 was significantly upregulated [169]. In DS, it is probable that BACH1 protein upregulation could block the induction of antioxidant genes, therefore eliciting increased oxidative stress in the cell [163].
The analyses of two more genes, S100β and Ets-2, both located on chromosome 21, have been reported as associated with oxidative damage.
S100β (S100 calcium-binding protein, beta; OMIM *176,990) is an astroglia-derived Ca2+-binding protein actively secreted from astrocytes that modulates the activity of neurons, microglia, astrocytes, monocytes, and endothelial cells depending on its concentration [170]. S100β increased expression in astrocytes from DS and Alzheimer’s patients was shown in association with neuritic plaques [171]. The overexpression of S100β increases ROS formation and results in increasing neuritic neuronal and APP with consequently accelerated amyloid accumulation [117].
Ets-2 (ETS Protooncogene 2, Transcription Factor) (OMIM *164,740) is a transcription factor playing crucial roles in immune responses, cancer, and bone development, and it is overexpressed in Down Syndrome [155]. Overexpression is associated with increased neuronal apoptosis [117]. Ets-2 overexpression in cultured HCN leads to activation of a mitochondrial death apoptotic pathway. In DS/AD brains, upregulation of ets-2 appears closely associated with AD neurodegenerative lesions. Chronic oxidative stress in DS and AD brains may promote ets-2 expression, which may predispose to the activation of a mitochondrial death pathway [172].
In conclusion, cognitive and neurological disorders may be the consequence of the overexpression of SOD1, APP, ETS-2, S100β, and abnormal production of BACH1. Finally, the following other main alterations that can affect individuals with DS are congenital and acquired cardiovascular abnormalities: although no specific gene has been identified, it seems that increased oxidative stress and mitochondrial dysfunction are associated with the increased development of these complications [173].
2.5. Marfan Syndrome
Marfan syndrome (MFS) is an autosomal dominant disease that affects the connective tissue with variable penetrance and an estimated prevalence of one in 10,000–20,000 individuals [188]. In 90% of cases [189], MFS is caused by mutations in Fibrillin-1 (FBN1), located on chromosome 15q21.1 and containing 65 exons [190]. The cardinal features involve the ocular and skeletal systems (e.g., tall stature, arachnodactyly, and ectopia lentis) [191], but the most life-threatening manifestations are related to cardiovascular complications, including mitral valve prolapse, arrhythmias, coronary artery disease, left ventricular hypertrophy, congestive heart failure, aortic insufficiency, dilatation of the aortic root, and aortic dissection [192,193]. Considering those cardiovascular complications, early recognition and appropriate management are critical for patients with MFS. Clinical criteria and, in particular, Ghent nosology, outlined in 2010, are used in the diagnosis of MFS [194].
It is well known that the highly reactive oxygen-derived free radicals (ROS) play an important role in the genesis and progression of various cardiovascular diseases, including arrhythmias, aortic dilatation, aortic dissection, left ventricular hypertrophy, coronary arterial disease, and congestive heart failure [195]. MFS is characterized by the presence of ascending aortic aneurysms resulting from the altered assembly of extracellular matrix fibrillin-containing microfibrils and dysfunction of TGF-β signaling [196]. It has been demonstrated that patients affected by MFS show impaired contractile function and endothelial-dependent relaxation resulting from oxidative stress in the thoracic aorta [197]. Endothelial dysfunction increases the inducible nitric oxide synthase (iNOS) pathway, leading to an excess in nitric oxide (NO) production that causes tissue damage [198]. Moreover, the results of studies have suggested that ROS could be involved in smooth muscle cell phenotype switching and apoptosis as well as matrix metalloproteinase activation, resulting in extracellular matrix (ECM) remodeling [199]. Among ROS species, Jiménez-Altayó et al. identified that H2O2 directly produced by NOX4 and/or by the transmutation of O2•− by SODs could be the most relevant ROS candidate for the Marfan-associated redox stress because unlike O2•−, H2O2 is permeable to cell membranes and has a significantly longer lifespan than O2•−, showing high reactivity for cysteine residues leading to their oxidation [200]. Oxidative stress plays an important role in the formation of the ascending aortic aneurysm but also in the evolution of the aneurysm. Studies on both animals and humans studies demonstrated that augmented redox stress is correlated to the progression of the aortic aneurysm [199]. According to some studies, the progression of thoracic aortic aneurysm is the result of the markedly impaired aortic contractile function as well as decreased nitric oxide (NO)-mediated endothelial-dependent relaxation [201]. Recently, Fiorillo et al. demonstrated, for the first time, signs of oxidative stress in the plasma of patients with MFS. Moreover, they showed a significant correlation between the intensity of oxidative stress and the severity of the clinical manifestations, suggesting systemic oxidative damage [202].
2.6. Fanconi’s Anemia
Fanconi’s anemia (FA) is an inherited pathology of DNA repair, with progressive pancytopenia, bone marrow failure, variable congenital malformations, and a predisposition to hematological or solid tumors [213]. AF is transmitted with an autosomal recessive inheritance and is due to mutations in genes involved in DNA repair and genomic stability. In total, 15 genes have been identified, these 15 genes encode proteins identified as FANCA, B, C, D1, D2, E, F, G, I, J, L, M, N, O, and P. These proteins form nuclear complexes and are activated in response to DNA damage breakage [214,215,216].
The incidence is approximately 1–5 cases per 1,000,000 inhabitants, with approximately 2000 cases described worldwide [213]. The phenotypic anomalies are many, such as aplasia of the radius, skin hyperpigmentation, microphthalmia, nystagmus, and reduced vision. Cardiac, renal, and urogenital defects and short stature, deafness, and hypogonadism can occur in a lower percentage of incidence [213]. The prognosis is unfavorable due to the higher incidence of solid tumors and leukemia [217].
In patients with FA, oxidative stress is an important determinant of some clinical conditions related to the disease. In fact, it has been seen that in affected patients there would be a reduction in the activity of SOD with consequently reduced production of antioxidant species. Furthermore, there would seem to be an increase in the production of TNF-α, which would lead to an increase in the production of O2•− with consequent apoptosis and disruption of DNA [218,219]. In fact, the increase in TNF-α would seem to lead to reduced activity of some proteins of the FANC group such as FANCA and FANCG with a reduced ability to prevent DNA damage [220]. The latter protein is expressed at the mitochondrial level and its mutation would lead to the reduced activation of a mitochondrial peroxide, PRDX3, with important antioxidant activities [219]. The FANCC protein, on the other hand, would bind cytochrome-P450 2E1 (CYP2E1), which has a detoxifying activity at the cellular level [220]. The FANCD2 protein interacts with the ATM protein and appears to stabilize it by reducing the production of radical species [218].
2.7. Autism Spectrum Disorders (ASD)
Autism spectrum disorders are defined as a group of neurodevelopmental disorders that involve alterations in social, work, school, and personal functioning [223]. They usually cause difficulties in acquiring, maintaining, and applying specific skills or sets of information [224]. The current prevalence in Italy is 15 children per 1000 with a 1:4 male-to-female ratio. In the last decade, there has been an increase in diagnoses due in part to the change in diagnostic criteria [225]. The etiopathogenesis is unknown and it is therefore believed that it may be multifactorial and dependent on both environmental and genetic factors. In recent years, the correlation between environmental pollutants and autism spectrum disorders has been studied, and what has emerged is that exposure to such substances as ionizing radiation, pesticides, and heavy metals increases the risk of developing this pathology [226]. From a clinical point of view, the symptoms are variable but usually characterized by the following: persistent deficits in social communication and interaction, repetitiveness, and sectorial behavior, interests, or activities [224]. The diagnosis is clinical and is based on the evaluation of the patient and on the execution of some tests that are generally carried out from the age of two, including the Autism Diagnostic Interview-Revised (ADI-R); Childhood Autism Rating Scale (CARS) and Autism Diagnostic Observation Schedule (ADOS) [225]. Regarding oxidative stress, patients with ASD seem to have higher levels than the general population [227]. This would seem to be mainly due to a reduction in the activity of endogenous antioxidants such as SOD, GTPx, and CAT [228]. The reduction of the enzymatic activity would seem to increase the production of pro-oxidizing and lipid peroxidation-related substances such as F2-isoprostane and 8-iso-prostaglandin F2α [229]. Furthermore, there would seem to be an alteration in metallothionein-3 (MT-3), a protein with detoxifying activity expressed in the brain. This, in fact, seems to be reduced in patients with ASD, causing greater neuronal toxicity [227]. It should also be mentioned that, in general, in patients with ASD, there is a reduction in total glutathione with consequently reduced detoxification of pro-oxidizing metabolites [229].
2.8. Primitive Immunodeficiencies
Primitive immunodeficiencies (PID) represent a wide group of diseases, generally considered as conditions with an increased rate of serious and recurring infections caused by an alteration in the immune response. In the immuno-dysregulated scenario, along with the augmented infection susceptibility, there is also a manifestation of autoimmunity. As said, under the definition of PID, there are numerous clinical syndromes [234]. However, the correlation between these syndromes and oxidative stress has been sufficiently studied only in a few of them, as follows: the common variable immunodeficiency disease (CVID), the most serious subtype of severe combined immunodeficiency (SCID), the reticular dysgenesis (RD), and the chronic granulomatous disease (CGD) [235].
2.9. Gaucher Disease
Gaucher disease (GD) is one of the most common lysosomal storage diseases and it is caused by mutations in the gene GBA1. GBA1 encodes the enzyme glucocerebrosidase (GCase, acid-β-glucosidase) that leads to altered glucocerebrosidase activity, resulting in the accumulation and storage of glycosphingolipids as the glucocerebroside (GL1) in the lysosomes of macrophages. GL1-engorged macrophages (Gaucher cells) are usually found in the bone marrow, spleen, liver, lungs, lymph nodes, and other organs of patients with GD [251,252].
Glucosylsphingosine (GlcSph, lyso-GL1, lyso-Gb1) is another glycosphingolipid that is present in much lower concentration but probably more pathogenic than GL1 itself in patients with GD [253]. There are the following three different subtypes of GD, according to the presence of neurological deterioration, age at identification, and disease progression rate: the most common is represented by GD1 (non-neuronopathic); GD2 (acute neuronopathic), and GD3 (chronic neuronopathic), which are less common but are associated with a more severe phenotype [254]. GD is normally suspected for the presence of unexpected anemia, thrombocytopenia, and organomegaly. The gold standard for the diagnosis of GD is the presence of low enzymatic activity of GBA1 in peripheral blood compared with normal controls [255,256].
There are the following four treatments available for GD1: 3 ERTs and 1 SRT. No drugs have been approved for GD2 or GD3 [251]. Despite the current therapies, chronic pain and fatigue are two of the most important symptoms associated with GD1. This is probably due to the chronic inflammation associated with the release of pro-inflammatory cytokines and other mediators either directly from Gaucher macrophages or indirectly by crosstalk between Gaucher cells and immunomodulatory lymphocytes [257]. However, it has been reported that the accumulation of toxic glycosphingolipids within cells leads to the production of reactive oxygen species (ROS) and an imbalance between the pro-oxidants and the antioxidant reserve, resulting in oxidative stress and inflammation [258].
The loss of the function of GCase is in fact responsible for an important decrease in the mitochondrial membrane potential, adenosine diphosphate phosphorylation, and an increase in oxidative stress and fragmentation of mitochondria [259]. For this reason, different authors have tried to study the relation between oxidative stress and GD. Rollo et al. investigated the relationship between ROS and GD by analyzing blood oxidative stress markers in GD patients submitted to ERT at different stages during the treatment. They discovered that RT-treated GD patients showed an improvement in antioxidant capacity, which was further increased just after recombinant enzyme infusion [260]. Mello et al. showed an alteration in the concentration of CAT, SOD, and SH, which suggests that there was a change in reactive oxygen species in GD type I patients when compared to HC. This increase in CAT, SOD, and sulfhydryl could likely be related to the prevention of the increase in hydrogen peroxide, preventing damage to lipids [258].
Recently, Kartha et al. decided to study the levels of multiple oxidative stress biomarkers in plasma and red blood cells from untreated patients, in stable individuals undergoing standard-of-care therapy, and in healthy controls.. They found significant differences in key oxidative stress biomarkers in untreated patients compared to healthy controls, while in treated patients, results generally fell between the controls and the untreated patients [255].
This entry is adapted from the peer-reviewed paper 10.3390/antiox11101983
This entry is offline, you can click here to edit this entry!
