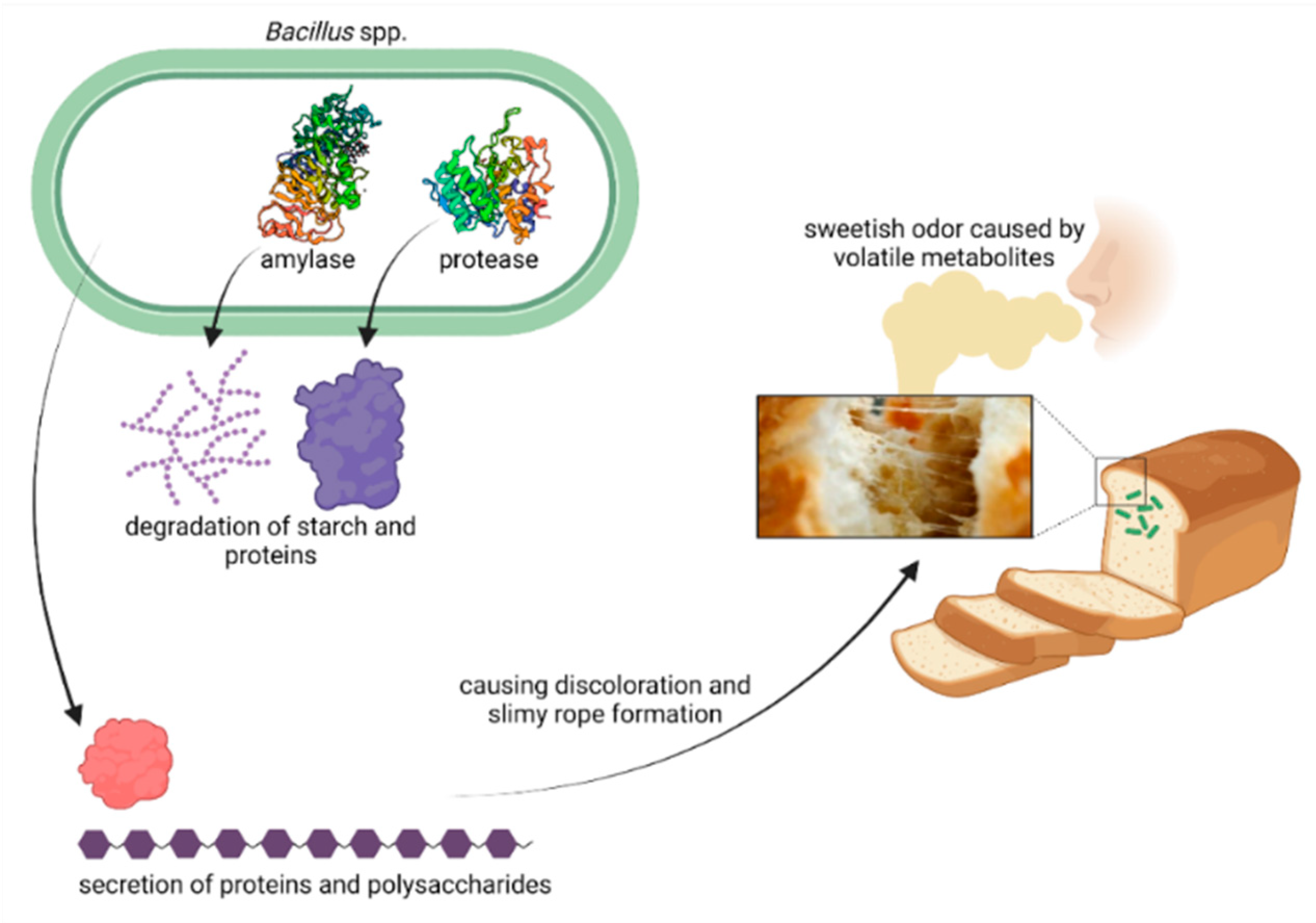
As bread is a very important staple food, its spoilage threatens global food security. Ropy bread spoilage manifests in sticky and stringy degradation of the crumb, slime formation, discoloration, and an odor reminiscent of rotting fruit. Increasing consumer demand for preservative-free products and global warming may increase the occurrence of ropy spoilage. Bacillus amyloliquefaciens, B. subtilis, B. licheniformis, the B. cereus group, B. pumilus, B. sonorensis, Cytobacillus firmus, Niallia circulans, Paenibacillus polymyxa, and Priestia megaterium were reported to cause ropiness in bread. To date, the underlying mechanisms behind ropy bread spoilage remain unclear, high-throughput screening tools to identify rope-forming bacteria are missing, and only a limited number of strategies to reduce rope spoilage were described.
- Bacillus spp.
- bread
- rope spoilage
- wheat
1. Introduction
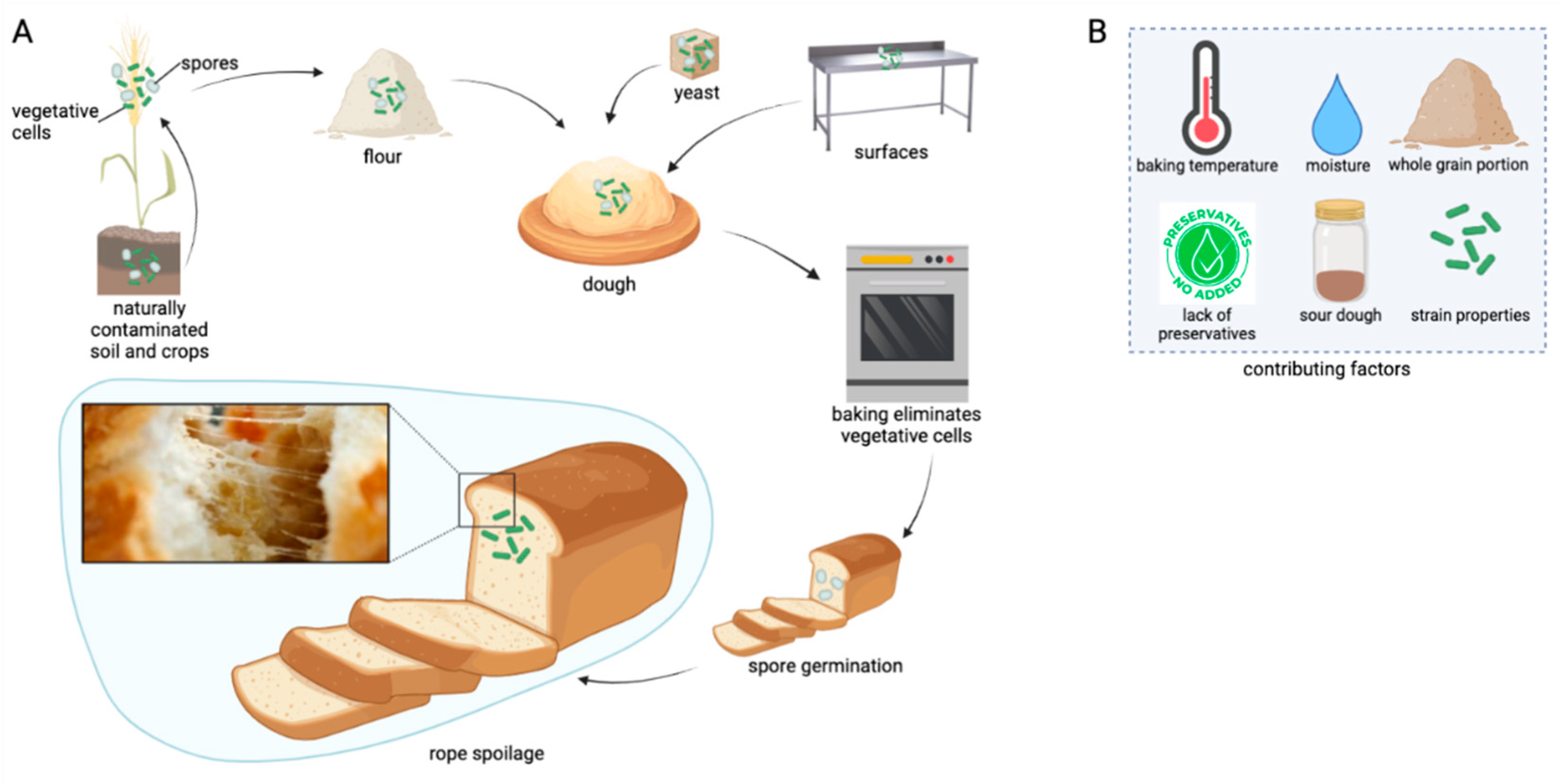
2. The Route of Endospores into the Bakery Environment
3. The Diversity of Species Inducing Rope Spoilage in Bread
Considering recent changes in taxonomy and the microbiological and molecular biological methodologies used, attribution of strains to some of the species might not be consistent with current taxonomic frameworks and must be interpreted with caution. Table 1 provides an overview over the different bacterial species associated with rope spoilage.
Table 1. Bacterial species that were suggested to cause rope spoilage in bread. The table summarizes data on growth characteristics, metabolism, and spore survival.
| Growth | Metabolism | Survival | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxonomy | Optimum Growth Temperature [°C] | Minimum Growth Temperature [°C] | Maximum Growth Temperature [°C] | Growth at pH | NaCl Tolerance | Anaerobic Growth | Urease | Nitrate Reduction | Hydrolysis of Starch | Citrate | Propionate | Egg Yolk Reaction | Spore D100 Value [min] | References |
| B. amyloliquefaciens | 30–40 | 15 | 50 | 5.7 | 5–10% | − | + | d | + | + | n.a. | + | 23–44 | [11][20][34][35][36][37][38][39] |
| B. cereus group | 37 | 5 | 50 | 4.9–9.3 | n.a. | + | d | + | + | d | n.a | + | <10 | [18][20][29][30][31][32][33][34][35][36][40][41][42][43][44][45][46] |
| B. licheniformis | 37 | 15 | 50–55 | 5.7 | 7% | + | + | + | + | + | + | − | 56 | [11][18][20][34][35][36][39][41][45][47][48][49] |
| B. pumilus | 30 | 15 | 50–55 | 5.7 | 7% | + | + | + | + | + | + | − | 56 | [11][18][20][34][35][36][39][41][45][47] |
| B. sonorensis | 30 | 15 | 55 | n.a. | <5% | + | + | + | + | + | + | − | n.a. | [41][45] |
| B. subtilisa | 28–30 | 5-20 | 45–55 | 5.5–8.5 | 7–10% | Facultative | − | + | + | + | − | − | 14 | [2][8][18][19][20][34][35][36][41][45][48][50][51][52] |
| C. firmus b | 30–37 | n.a. | n.a. | n.a. | n.a. | n.a. | − | + | + | − | n.a. | n.a. | n.a. | [34][35][36] |
| N. circulans c | 30–37 | n.a. | n.a. | n.a. | n.a. | n.a. | − | n.a. | + | − | n.a. | n.a. | n.a. | [34][35][36] |
| P. polymyxa d | 30 | n.a. | n.a. | n.a. | n.a. | n.a. | − | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | [20][41][45] |
| Pr. megateriume | 30 | 3-15 | 35–45 | n.a. | 7% | − | + | d | + | + | n.a. | − | n.a. | [20][36][47] |
4. Prevention of Rope Formation in Bread
5. Considerations for the Future
This entry is adapted from the peer-reviewed paper 10.3390/foods11193021
References
- James P. Smith; Daphne Phillips Daifas; Wassim El-Khoury; John Koukoutsis; Anis El-Khoury; Shelf Life and Safety Concerns of Bakery Products—A Review. Critical Reviews in Food Science and Nutrition 2004, 44, 19-55, 10.1080/10408690490263774.
- A. J. Amos; D. W. Kent-Jones; The “rope” spore content of flour and its significance. The Analyst 1931, 56, 572-586, 10.1039/an9315600572.
- Saranraj, P.; Sivasakthivelan, P.. Microorganisms involved in spoilage of bread and its control measures. In Bread and Its Fortification: Nutrition and Health Benefits; Rosell, C.M., Bajerska, J., El Sheikha, A.F., Eds.; CRC Press: Boca Raton: FL, USA, 2015; pp. 132–149.
- E. J. Cohn; S. B. Wolbach; L. J. Henderson; P. H. Cathcart; ON THE CONTROL OF ROPE IN BREAD. Journal of General Physiology 1918, 1, 221-230, 10.1085/jgp.1.2.221.
- Cook, F.K.; Johnson, B.L.. Ropiness in flour and bread and its detection and prevention. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W.H., Doyle, M.P., Eds.; Springer : New York, NY, USA; London, UK,, 2009; pp. 223–244.
- Claudia Axel; Emanuele Zannini; Elke K. Arendt; Mold spoilage of bread and its biopreservation: A review of current strategies for bread shelf life extension. Critical Reviews in Food Science and Nutrition 2017, 57, 3528-3542, 10.1080/10408398.2016.1147417.
- Mahfuzur Rahman; Senay Simsek; Go clean label: replacement of commercial dough strengtheners with hard red spring wheat flour in bread formulations. Journal of Food Science and Technology 2020, 57, 3581-3590, 10.1007/s13197-020-04390-w.
- Logan, N.A.; De Vos, P. . Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–163.
- Graham Christie; Peter Setlow; Bacillus spore germination: Knowns, unknowns and what we need to learn. Cellular Signalling 2020, 74, 109729, 10.1016/j.cellsig.2020.109729.
- Kanika Khanna; Javier Lopez-Garrido; Kit Pogliano; Shaping an Endospore: Architectural Transformations During Bacillus subtilis Sporulation. Annual Review of Microbiology 2020, 74, 361-386, 10.1146/annurev-micro-022520-074650.
- Lavermicocca, P.; Valerio, F.; DeBellis, P.; Sisto, A.; Leguérinel, I. Chapter 16—Sporeforming bacteria associated with bread production: Spoilage and toxigenic potential. In Food Hygiene and Toxicology in Ready to Eat Foods; Kotzekidou, P., Eds.; Academic Press (Elsevier): Amsterdam, The Netherlands, 2016; pp. 275–293.
- Cauvain, S.. Technology of Breadmaking, 3rd ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1-408.
- Stéphane André; Tatiana Vallaeys; Stella Planchon; Spore-forming bacteria responsible for food spoilage. Research in Microbiology 2017, 168, 379-387, 10.1016/j.resmic.2016.10.003.
- F. J. Farmiloe; S. J. Cornford; J. B. M. Coppock; M. Ingram; The survival ofBacillus subtilis spores in the baking of bread. Journal of the Science of Food and Agriculture 1954, 5, 292-304, 10.1002/jsfa.2740050608.
- Jackie M. Thompson; Christine E.R. Dodd; Will M. Waites; Spoilage of bread by bacillus. International Biodeterioration & Biodegradation 1993, 32, 55-66, 10.1016/0964-8305(93)90039-5.
- J.M. Thompson; W.M. Waites; C.E.R. Dodd; Detection of rope spoilage in bread caused by Bacillus species. Journal of Applied Microbiology 1998, 85, 481-486, 10.1046/j.1365-2672.1998.853512.x.
- James P. Smith; Daphne Phillips Daifas; Wassim El-Khoury; John Koukoutsis; Anis El-Khoury; Shelf Life and Safety Concerns of Bakery Products—A Review. Critical Reviews in Food Science and Nutrition 2004, 44, 19-55, 10.1080/10408690490263774.
- H Rosenkvist; Contamination profiles and characterisation of Bacillus species in wheat bread and raw materials for bread production. International Journal of Food Microbiology 1995, 26, 353-363, 10.1016/0168-1605(94)00147-x.
- Olimpia Pepe; Giuseppe Blaiotta; Giancarlo Moschetti; Teresa Greco; Francesco Villani; Rope-Producing Strains of Bacillus spp. from Wheat Bread and Strategy for Their Control by Lactic Acid Bacteria. Applied and Environmental Microbiology 2003, 69, 2321-2329, 10.1128/aem.69.4.2321-2329.2003.
- F. Valerio; P. De Bellis; M. Di Biase; S.L. Lonigro; B. Giussani; A. Visconti; P. Lavermicocca; A. Sisto; Diversity of spore-forming bacteria and identification of Bacillus amyloliquefaciens as a species frequently associated with the ropy spoilage of bread. International Journal of Food Microbiology 2012, 156, 278-285, 10.1016/j.ijfoodmicro.2012.04.005.
- Rani, U.; Sharma, S.; Kumar, V. . Bacillus Species: A Potential Plant Growth Regulator. In Bacilli and Agrobiotechnology: Phytostimulation and Biocontrol; Islam, M.T., Rahman, M.M., Pandey, P., Boehme, M.H., Haesaert, G., Eds.; Springer: Cham, Switzerland, 2019; pp. 29–47.
- Frédéric Carlin; Origin of bacterial spores contaminating foods. Food Microbiology 2011, 28, 177-182, 10.1016/j.fm.2010.07.008.
- Agata Los; Dana Ziuzina; Paula Bourke; Current and Future Technologies for Microbiological Decontamination of Cereal Grains. Journal of Food Science 2018, 83, 1484-1493, 10.1111/1750-3841.14181.
- Ana M. Magallanes López; Senay Simsek; Pathogens control on wheat and wheat flour: A review. Cereal Chemistry 2020, 98, 17-30, 10.1002/cche.10345.
- Luis Sabillón; Andréia Bianchini; From Field to Table: A Review on the Microbiological Quality and Safety of Wheat-Based Products. Cereal Chemistry 2016, 93, 105-115, 10.1094/cchem-06-15-0126-rw.
- Holzapfel, P.. Zerealien und Nährmittel. In Handbuch Lebensmittelhygiene: Praxisleitfaden mit Wissenschaftlichen Grundlagen; Alter, T., Kleer, J., Kley, F., Eds.; Behr: Hamburg, Germany, 2005; pp. XIII.5–XII.6..
- Lana K Berghofer; Ailsa D Hocking; Di Miskelly; Edward Jansson; Microbiology of wheat and flour milling in Australia. International Journal of Food Microbiology 2002, 85, 137-149, 10.1016/s0168-1605(02)00507-x.
- Luis Sabillón; Jayne Stratton; Devin J. Rose; Teshome Regassa; Andréia Bianchini; Microbial Load of Hard Red Winter Wheat Produced at Three Growing Environments across Nebraska, USA. Journal of Food Protection 2016, 79, 646-654, 10.4315/0362-028x.jfp-15-424.
- Yun-Xia Chen; Xiao-Na Guo; Jun-Jie Xing; Xiao-Hong Sun; Ke-Xue Zhu; Effects of wheat tempering with slightly acidic electrolyzed water on the microbial, biological, and chemical characteristics of different flour streams. LWT 2020, 118, 108790, 10.1016/j.lwt.2019.108790.
- Serra, S.; De Simeis, D.; New insights on the baker’s yeast-mediated hydration of oleic acid: The bacterial contaminants of yeast are responsible for the stereoselective formation of (R)-10-hydroxystearic acid. Journal of applied microbiology 2017, 124, 719–729, 10.1111/jam.13680.
- Gélinas, P.; Mapping Patents on Post-Fermentation Processes.. Comprehensive Reviews in Food Science and Food Safety 2017, 16, 456–476, 10.1111/1541-4337.12256.
- Clint R. Viljoen; Alexander von Holy; Microbial populations associated with commercial bread production. Journal of Basic Microbiology 1997, 37, 439-444, 10.1002/jobm.3620370612.
- C.P. Bailey; A. Von Holy; Bacillus spore contamination associated with commercial bread manufacture. Food Microbiology 1993, 10, 287-294, 10.1006/fmic.1993.1033.
- Collins, N.E.; Ann, L.; Kirschner, M.; von Holy, A.; Characterization of Bacillus isolates from ropey bread, bakery equipment and raw materials. South African Journal of Science 1991, 87, 62–66, .
- Aidan C. Parte; Joaquim Sardà Carbasse; Jan P. Meier-Kolthoff; Lorenz C. Reimer; Markus Göker; List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. International Journal of Systematic and Evolutionary Microbiology 2020, 70, 5607-5612, 10.1099/ijsem.0.004332.
- Radhey S. Gupta; Sudip Patel; Navneet Saini; Shu Chen; Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. International Journal of Systematic and Evolutionary Microbiology 2020, 70, 5753-5798, 10.1099/ijsem.0.004475.
- Jia, Y.; Fang, F.; Improving applicability of urease from Bacillus amyloliquefaciens JP-21 by site-directed mutagenesis.. Chinese Journal of Biotechnology 2020, 36, 1640–1649, 10.13345/j.cjb.190566.
- Y.S. Kim; K. Balaraju; Y.H. Jeon; Biological characteristics ofBacillus amyloliquefaciensAK-0 and suppression of ginseng root rot caused byCylindrocarpon destructans. Journal of Applied Microbiology 2016, 122, 166-179, 10.1111/jam.13325.
- Leuschner, R.; O’Callaghan, M.; Arendt, E.; Bacilli Spoilage in Part-baked and Rebaked Brown Soda Bread. Journal of food science 1998, 63, 915–918, 10.1111/j.1365-2621.1998.tb17926.x.
- Adriana Laca; Zoe Mousia; Mario Dı́az; Colin Webb; Severino S. Pandiella; Distribution of microbial contamination within cereal grains. Journal of Food Engineering 2006, 72, 332-338, 10.1016/j.jfoodeng.2004.12.012.
- Ana Paula M. Pereira; Graziele C. Stradiotto; Luísa Freire; Verônica O. Alvarenga; Aline Crucello; Letícia L.P. Morassi; Fabiana P. Silva; Anderson S. Sant’Ana; Occurrence and enumeration of rope-producing spore forming bacteria in flour and their spoilage potential in different bread formulations. LWT 2020, 133, 110108, 10.1016/j.lwt.2020.110108.
- J.E. Dexter; P.J. Wood; Recent applications of debranning of wheat before milling. Trends in Food Science & Technology 1996, 7, 35-41, 10.1016/0924-2244(96)81326-4.
- Reale, A.; Di Renzo, T.; Succi, M.; Tremonte, P.; Coppola, R.; Sorrentino, E.; Microbiological and Fermentative Properties of Baker’s Yeast Starter Used in Breadmaking. Journal of food science 2013, 78, M1224–M1231, 10.1111/1750-3841.12206.
- Viljoen, B.; Lues, J.; The microbial populations associated with post-fermented dough and compressed baker’s yeast.. Food microbiology 1993, 10, 379–386, 10.1006/fmic.1993.1044.
- Min-Jung Kwak; Seon-Bin Choi; Sung-Min Ha; Eun Hye Kim; Byung-Yong Kim; Jongsik Chun; Genome-based reclassification of Paenibacillus jamilae Aguilera et al. 2001 as a later heterotypic synonym of Paenibacillus polymyxa (Prazmowski 1880) Ash et al. 1994. International Journal of Systematic and Evolutionary Microbiology 2020, 70, 3134-3138, 10.1099/ijsem.0.004140.
- Maarten Mols; Tjakko Abee; Role of Ureolytic Activity in Bacillus cereus Nitrogen Metabolism and Acid Survival. Applied and Environmental Microbiology 2008, 74, 2370-2378, 10.1128/aem.02737-07.
- Fatma M. Helmi; Hemdan R. Elmitwalli; Sherif M. Elnagdy; Abeer F. El-Hagrassy; Calcium carbonate precipitation induced by ureolytic bacteria Bacillus licheniformis. Ecological Engineering 2016, 90, 367-371, 10.1016/j.ecoleng.2016.01.044.
- I.B. Sorokulova; O.N. Reva; V.V. Smirnov; I.V. Pinchuk; S.V. Lapa; M.C. Urdaci; Genetic diversity and involvement in bread spoilage of Bacillus strains isolated from flour and ropy bread.. Letters in Applied Microbiology 2003, 37, 169-173, 10.1046/j.1472-765x.2003.01372.x.
- Ali Vahabi; Ali Akbar Ramezanianpour; Hakimeh Sharafi; Hossein Shahbani Zahiri; Hojatollah Vali; Kambiz Akbari Noghabi; Calcium carbonate precipitation by strainBacillus licheniformisAK01, newly isolated from loamy soil: a promising alternative for sealing cement-based materials. Journal of Basic Microbiology 2013, 55, 105-111, 10.1002/jobm.201300560.
- Dykes, G.A.; Kirschner, L.M.; von Holy, A; Differentiation of Bacillus isolates from ropey bread and the bakery environment using numerical taxonomy. South African Journal of Science 1994, 90, 302–307, https://hdl.handle.net/10520/AJA00382353_5198.
- Christopher A. Dunlap; Michael J. Bowman; Daniel R. Zeigler; Promotion of Bacillus subtilis subsp. inaquosorum, Bacillus subtilis subsp. spizizenii and Bacillus subtilis subsp. stercoris to species status. Antonie van Leeuwenhoek 2019, 113, 1-12, 10.1007/s10482-019-01354-9.
- Mengmei Ma; Taihua Mu; Liang Zhou; Identification of saprophytic microorganisms and analysis of changes in sensory, physicochemical, and nutritional characteristics of potato and wheat steamed bread during different storage periods. Food Chemistry 2020, 348, 128927, 10.1016/j.foodchem.2020.128927.
- Lina Vaiciulyte-Funk; Renata Žvirdauskienė; Joana Šalomskienė; Antanas Sarkinas; The effect of wheat bread contamination by the Bacillus genus bacteria on the quality and safety of bread. Zemdirbyste-Agriculture 2015, 102, 351-358, 10.13080/z-a.2015.102.045.
- T.L. Pattison; D. Lindsay; A. von Holy; In vitro growth response of bread-spoilage Bacillus strains to selected natural antimicrobials. Journal of Basic Microbiology 2003, 43, 341-347, 10.1002/jobm.200390037.
- Vera Fraberger; Christine Unger; Christian Kummer; Konrad J. Domig; Insights into microbial diversity of traditional Austrian sourdough. LWT 2020, 127, 109358, 10.1016/j.lwt.2020.109358.
- Pilar Martínez Viedma; Hikmate Abriouel; Nabil Ben Omar; Rosario Lucas López; Antonio Gálvez; Inhibition of spoilage and toxigenic Bacillus species in dough from wheat flour by the cyclic peptide enterocin AS-48. Food Control 2010, 22, 756-761, 10.1016/j.foodcont.2010.11.010.
- A. Digaitiene; Åse Solvej Hansen; G. Juodeikiene; D. Eidukonyte; J. Josephsen; Lactic acid bacteria isolated from rye sourdoughs produce bacteriocin-like inhibitory substances active against Bacillus subtilis and fungi. Journal of Applied Microbiology 2012, 112, 732-742, 10.1111/j.1365-2672.2012.05249.x.
- Vera Fraberger; Claudia Ammer; Konrad J. Domig; Functional Properties and Sustainability Improvement of Sourdough Bread by Lactic Acid Bacteria. Microorganisms 2020, 8, 1895, 10.3390/microorganisms8121895.
- Ioanna Mantzourani; Stavros Plessas; Maria Odatzidou; Athanasios Alexopoulos; Alex Galanis; Eugenia Bezirtzoglou; Argyro Bekatorou; Effect of a novel Lactobacillus paracasei starter on sourdough bread quality. Food Chemistry 2018, 271, 259-265, 10.1016/j.foodchem.2018.07.183.
- Stavros Plessas; Ioanna Mantzourani; Argyro Bekatorou; Evaluation of Pediococcus pentosaceus SP2 as Starter Culture on Sourdough Bread Making. Foods 2020, 9, 77, 10.3390/foods9010077.
- Aliona Ghendov-Mosanu; Elena Cristea; Antoanela Patras; Rodica Sturza; Silvica Padureanu; Olga Deseatnicova; Nadejda Turculet; Olga Boestean; Marius Niculaua; Potential Application of Hippophae Rhamnoides in Wheat Bread Production. Molecules 2020, 25, 1272, 10.3390/molecules25061272.
- Ioanna Mantzourani; Antonia Terpou; Athanasios Alexopoulos; Eugenia Bezirtzoglou; Stavros Plessas; Assessment of Ready-to-Use Freeze-dried Immobilized Biocatalysts as Innovative Starter Cultures in Sourdough Bread Making. Foods 2019, 8, 40, 10.3390/foods8010040.
- Francesca Valerio; Palmira De Bellis; Stella L. Lonigro; Angelo Visconti; Paola Lavermicocca; Use of Lactobacillus plantarum fermentation products in bread-making to prevent Bacillus subtilis ropy spoilage. International Journal of Food Microbiology 2008, 122, 328-332, 10.1016/j.ijfoodmicro.2008.01.005.
- Özay Menteş; Recai Ercan; Mustafa Akçelik; Inhibitor activities of two Lactobacillus strains, isolated from sourdough, against rope-forming Bacillus strains. Food Control 2007, 18, 359-363, 10.1016/j.foodcont.2005.10.020.
- K Katina; M Sauri; H.-L Alakomi; T Mattila-Sandholm; Potential of Lactic Acid Bacteria to Inhibit Rope Spoilage in Wheat Sourdough Bread. LWT 2002, 35, 38-45, 10.1006/fstl.2001.0808.
- L G Eliseeva; D S Kokorina; E V Zhirkova; S A Smirova; E V Nevskaya; The Quality and Microbiological Stability of Quinoa-enriched Wheat Bread. IOP Conference Series: Earth and Environmental Science 2021, 670, 012020, 10.1088/1755-1315/670/1/012020.
- I. Mantzourani; S. Plessas; G. Saxami; A. Alexopoulos; A. Galanis; E. Bezirtzoglou; Study of kefir grains application in sourdough bread regarding rope spoilage caused by Bacillus spp.. Food Chemistry 2014, 143, 17-21, 10.1016/j.foodchem.2013.07.098.
- Carola Bücher; Johanna Burtscher; Konrad J. Domig; Propionic acid bacteria in the food industry: An update on essential traits and detection methods. Comprehensive Reviews in Food Science and Food Safety 2021, 20, 4299-4323, 10.1111/1541-4337.12804.
- M.L. Sudha; P. Viswanath; V. Siddappa; S. Rajarathnam; M.N. Shashirekha; Control of rope spore forming bacteria using carambola (Averrhoa carambola) fruit pomace powder in wheat bread preparation. Quality Assurance and Safety of Crops & Foods 2016, 8, 555-564, 10.3920/qas2014.0409.
- Zhen Li; Francieli Begnini Siepmann; Luis E. Rojas Tovar; Xiaoyan Chen; Michael G. Gänzle; Effect of copy number of the spoVA2mob operon, sourdough and reutericyclin on ropy bread spoilage caused by Bacillus spp.. Food Microbiology 2020, 91, 103507, 10.1016/j.fm.2020.103507.
- Arícia Possas; Antonio Valero; Fernando Pérez-Rodríguez; New software solutions for microbiological food safety assessment and management. Current Opinion in Food Science 2022, 44, 100814, 10.1016/j.cofs.2022.100814.
- E. Stavropoulou; E. Bezirtzoglou; Predictive Modeling of Microbial Behavior in Food. Foods 2019, 8, 654, 10.3390/foods8120654.
- Virginie Desvignes; Tasja Buschhardt; Laurent Guillier; Moez Sanaa; Quantitative microbial risk assessment for Salmonella in eggs. Food Modelling Journal 2019, 1, e39643, 10.3897/fmj.1.39643.
- Aidin Pahlavan; Mohammad Hassan Kamani; Amir Hossein Elhamirad; Zahra Sheikholeslami; Mohammad Armin; Hanieh Amani; Rapid quality assessment of bread using developed multivariate models: A simple predictive modeling approach. Progress in Agricultural Engineering Sciences 2020, 16, 1-10, 10.1556/446.2020.00001.
- Renata Różyło; Janusz Laskowski; PREDICTING BREAD QUALITY (BREAD LOAF VOLUME AND CRUMB TEXTURE). Polish Journal of Food and Nutrition Sciences 2011, 61, 61-67, 10.2478/v10222-011-0006-8.
- Gangawane, K.M.; Dwivedi, M.. Advanced Computational Techniques for Heat and Mass Transfer in Food Processing, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1-314 .
- C. Qian; N.H. Martin; M. Wiedmann; A. Trmčić; Development of a risk assessment model to predict the occurrence of late blowing defect in Gouda cheese and evaluate potential intervention strategies. Journal of Dairy Science 2022, 105, 2880-2894, 10.3168/jds.2021-21206.
