Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Chitosan Nanoparticles are made from chitosan or its derivatives. The N-deacetylated derivative of chitin is an appealing biopolymer for producing nanoparticles because chitosan has a unique polymeric cationic nature, non-toxicity, high biocompatibility, mucoadhesive properties, absorption-enhancing qualities, and biodegradability.
- drug delivery
- nanocarrier
- target therapy
- chemotherapy
- gene therapy
1. Introduction
ChNP boost the capacity of bioactive compounds to dissolve, entrap, encapsulate, and/or cling to the nanoparticle matrix. These systems have large surface areas where bioactives can be adsorbed. Their nanoscale size also improves efficient penetration through epitheliums. ChNP can also carry drugs, proteins, and DNA with low-to-high molecular weights and are negatively charged for targeting organs, cells, and tissues [1]. ChNP are also suitable for mucosal distribution, such as nasal, oral, and ocular mucosa, due to their characteristics and functions. When chitosan encounters anions, it forms a gel and beads and this feature allows it to be used in drug delivery. In addition, the size of the beads (1–2 mm) restricts its applicability [2][3]. Ohya and colleagues described ChNP for the first time in 1994 and employed emulsified and cross-linked ChNP to deliver the antitumor drug 5-fluorouracil intravenously [4][5]. To date, different techniques have been developed to produce ChNP and some of them are briefly discussed herein [6]. Overall, the most common techniques are ionotropic gelation and polyelectrolyte complexation since they are straightforward and do not need large shear forces or organic solvents [7].
2. Ionotropic Gelation
Chitosan can be cross-linked physically and chemically to generate nanoparticles because its backbone contains a number of amine groups that are protonated to form NH3+ in acidic conditions [8]. Physical cross-linking has sparked a lot of attention over chemical cross-linking as it eliminates toxic substances, reduces undesirable effects, and improves biocompatibility [9][10]. In addition, this simple and mild procedure allows for real cross-linking [11]. Physical cross-linking depends on the chitosan (positive charge) and multivalent ions (negative charge) generated by sodium tripolyphosphate (TPP) [12], citrate, and sulphate [13]. Calvo et al. were the first to report this ionic gelation process, which has been extensively studied and refined [14]. When chitosan gelation takes place by tiny anionic molecules, such as phosphate, citrate, or sulphate, the designated ionic gelation is used. When anionic macromolecules are used instead of tiny molecules, a polyelectrolyte complexion is thought to occur [15]. This approach makes use of the electrostatic interaction among the amine group of chitosan and a negatively charged polyanion such as tripolyphosphate [16]. Chitosan is poured into an acetic acid solution or added with a stabilising agent, such as poloxamer, and the tripolyphosphate aqueous solution is mixed under vigorous stirring. Then, anionic particles diffuse into the chitosan molecules and cross-linking occurs, leading to nanoparticle formation with a size range of 200–1000 nm, as shown in Figure 1A. After a couple of centrifugations and washing with water, ChNP are collected by freeze-drying or oven-drying. In this process, the chitosan-to-stabiliser ratio can alter the nanoparticles’ surface charge and size [17][18][19]. Increasing the chitosan-to-polyanion ratio results in an increase in particle size [20]. Since the smaller particle size was revealed in sodium chloride, nanoparticles disseminated in saline solution were also found to be more stable. The electrostatic repulsion between the amine groups of the chitosan backbone is reduced when a monovalent salt (sodium chloride) is added to the solvent. The polymer chains become more flexible, enhancing their stability [21].
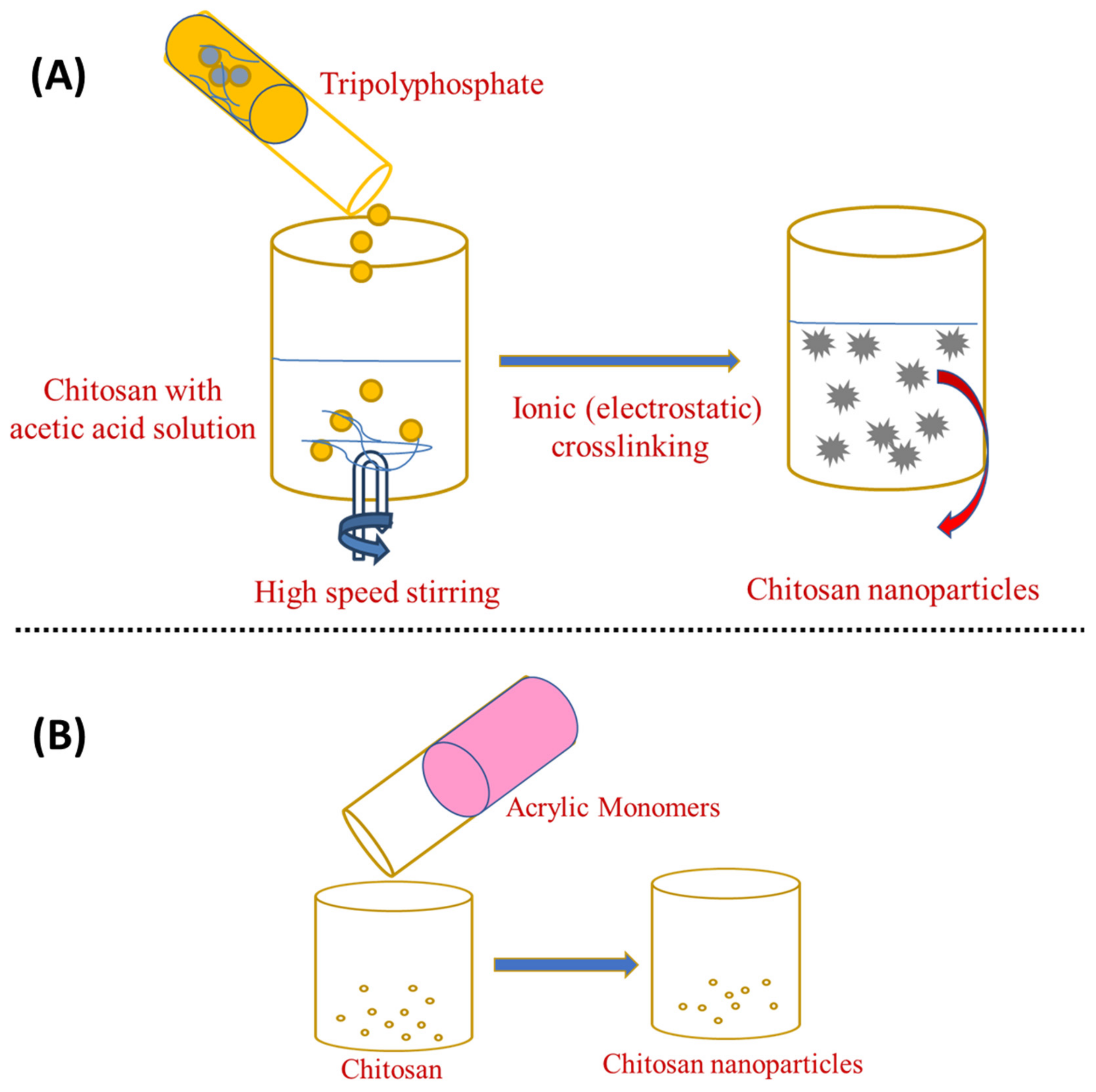
Figure 1. Preparation of chitosan nanoparticles by (A) ionotropic gelation and (B) ionotropic gelation with the radical polymerisation method.
Ionotropic gelation can also be combined with radical polymerisation, which causes chitosan to gel, whereas acrylic or methacrylic acid is polymerised [22]. As shown in Figure 1B, potassium persulfate is used as an initiator for the polymerisation reaction, which takes around 6 h to complete at 60–70 °C [16]. This approach eliminates the unreacted particles by dialysis or washing with water. Silk peptide, insulin, and serum albumin have been successfully loaded using this approach [23]. Overall, the ionotropic gelation method is the simplest and most cost-effective from bench-to-industrial scale-up. This is because this method requires simple, inexpensive materials and equipment and it can be easily accomplished moderately and rapidly in conventional research labs. Furthermore, the framework premised on electrostatic interaction rather than chemical reaction eradicates the need for organic solvents, avoiding unnecessary toxicological effects. Encapsulation efficiency can also be improved when the technique is set up to achieve optimised polymer–drug interactions [7].
3. Emulsion Droplet Coalescence and Emulsion Solvent Diffusion
In the emulsion droplet coalescence, a stable water-in-oil emulsion is made by homogenising an aqueous chitosan solution with the drug in liquid paraffin including a stabiliser, such as SpanTM 83, at high speed. During mixing both emulsions, droplets from both emulsions will collide and coalesce, causing chitosan droplets to precipitate and form nanoparticles of about 452 nm, as shown in Figure 2A [24].
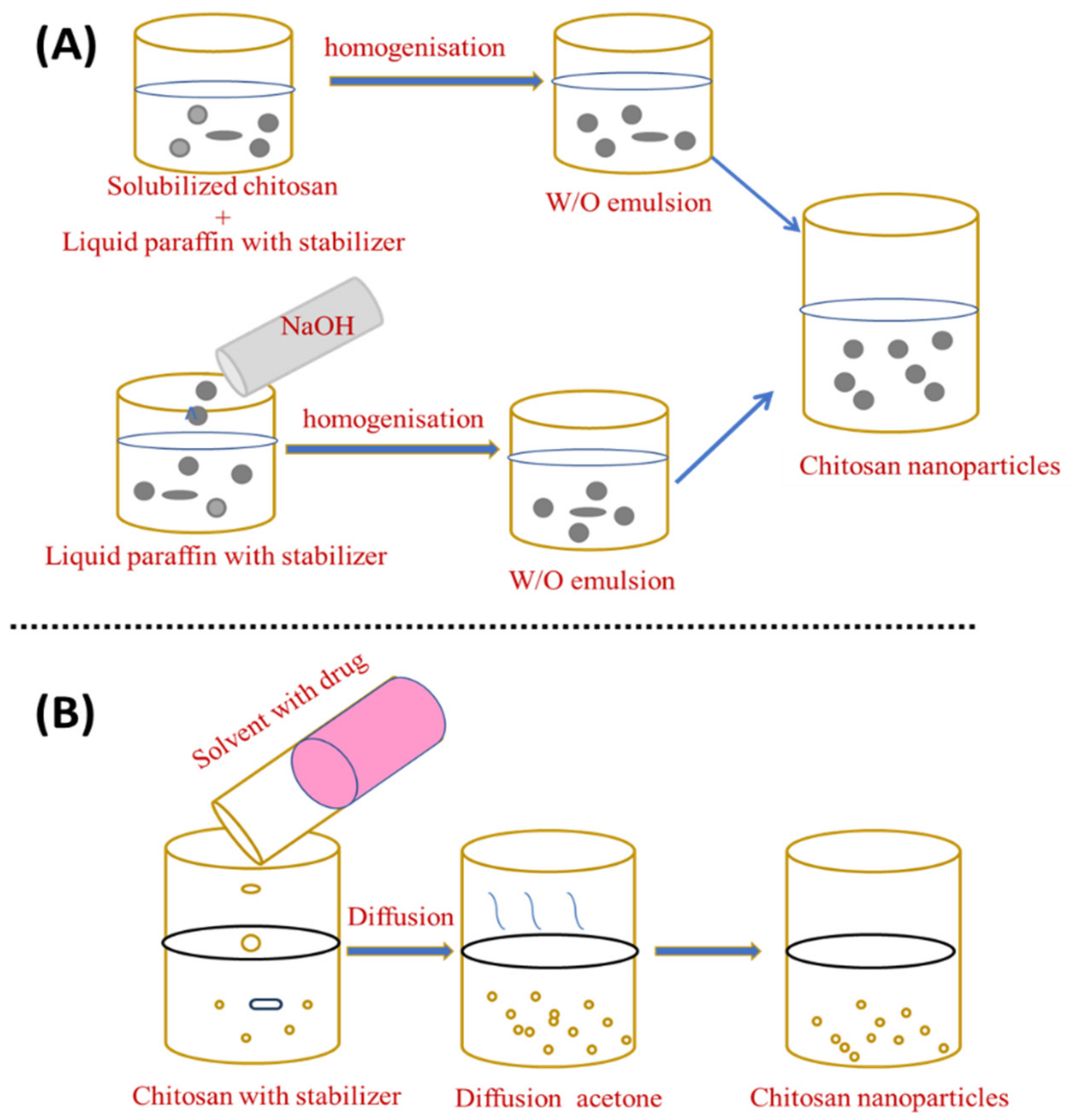
Figure 2. Preparation of chitosan nanoparticles by (A) emulsion droplet coalescence and (B) emulsion solvent diffusion.
El-Shabouri (2002) was the first to report on the emulsion solvent diffusion method [25] using a modified approach established by Niwa et al. [26]. Initially, an oil-in-water type emulsion is created by injecting an organic phase, such as acetone or methylene chloride, into a solution containing the hydrophilic drug to an aqueous chitosan solution containing a stabilising agent, such as lecithin/poloxamer, with stirring followed by high-pressure homogenisation to evaporate methylene chloride. By diffusing acetone into the aqueous phase, the solubility of chitosan is decreased and the polymer precipitates, owing to the organic solvent diffusion into the water, resulting in nanoparticles with an average size of between 100 and 500 nm [27], as shown in Figure 2B. An excess amount of water is added to ensure complete acetone diffusion and the nanoparticles are separated using centrifugation. This novel approach can reduce the particle size and distribution of the synthesised ChNP. Although the emulsification strategy leads to better particle size control, strong cross-linking agents are generally used in this procedure and the total elimination of the residual cross-linking agents can be challenging [28]. The emulsion solvent diffusion method is appropriate for both hydrophobic and hydrophilic drugs. In the case of hydrophilic drugs, a multiple water/oil/water emulsion (e.g., double emulsion) with the drug dissolved in the internal aqueous phase should be established. However, the use of high shear forces and organic solvents required during nanoparticle formation are two drawbacks of this approach.
4. Reverse Micellar Method
The reverse micellar approach is used to produce polymeric nanoparticles with a narrow size distribution [26]. Different polymers can be used to obtain micelles to carry drugs. Reverse micelles are thermodynamically stable liquid mixtures of water, oil, and surfactant. Compared to the typical emulsion polymerisation processes, the reverse micelle-hosted technology has a dynamic behaviour due to the generation of particles with a narrow size range [9]. This method uses a surfactant dissolved in an organic solvent to create reverse micelles. The Brownian motion randomly shifts the micellar droplets so they divide into two micelles after swapping their water content [29].
To prepare an organic phase, a lipophilic surfactant, such as sodium bis-(2-ethylhexyl) sulphosuccinate, is mixed in an organic solvent such as n-hexane (water in oil emulsion). After that, an aqueous drug–chitosan solution is added to the surfactant and organic solvent while stirring. After adding a cross-linking agent, the liquid is stirred overnight for cross-linking. Then, the organic solvent is eliminated, leaving a dry substance. The dried material is added to water to eliminate the surfactant and salt is applied to remove the surfactant, followed by centrifugation to recover the ChNP. The isolation of the nanoparticles is accomplished in three steps: surfactant precipitation with CaCl2, dialysis to remove unreacted components, and freeze-drying [30]. This allows the production of small, narrow particle sizes. The steps in this strategy are depicted in Figure 3A. Bovine serum albumin (BSA)-loaded ChNP in the 80–180 nm size range were prepared using the reverse micellar technique [31].
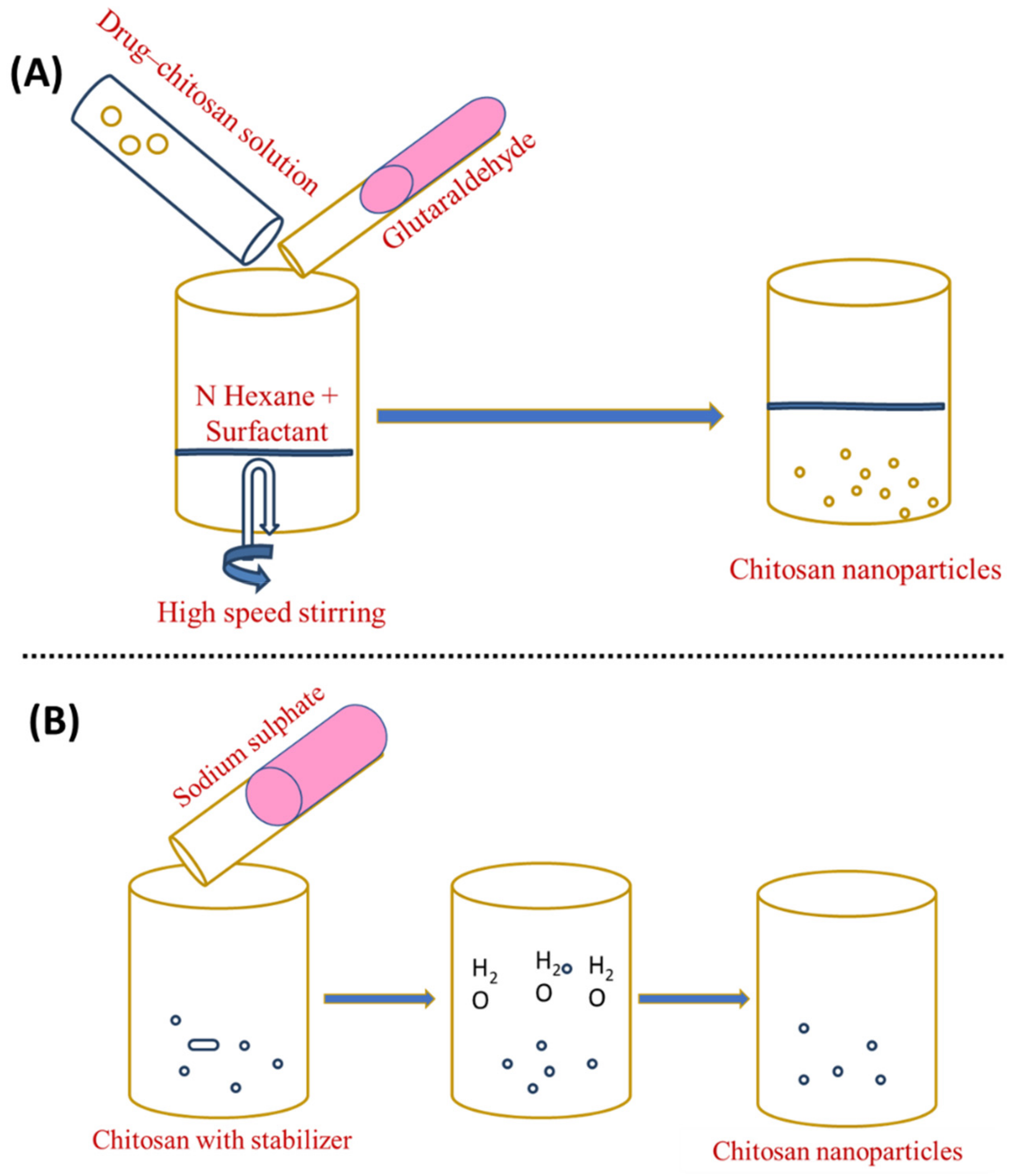
Figure 3. Preparation of chitosan nanoparticles by (A) reverse micellisation and (B) desolvation.
5. Desolvation
The desolvation process was employed for the first time to manufacture micron-sized carriers [6]. Chitosan nanoparticles are frequently made using a process modified from sodium sulphate [32]. DNA and protein distribution is possible using this method [33]. Phase separation and coacervation are the foundations of the desolvation technique. Nanoparticles precipitate when desolvation agents (such as ethanol or acetone) are added, and nanoparticle stability is achieved by adding a cross-linking agent [34]. In this process, an aqueous chitosan solution with a stabilising agent (e.g., Tween 20) is treated with a precipitation agent (e.g., sodium sulphate). The salty chitosan solution causes a steady removal of water-encircling chitosan. Due to the insolubilisation of chitosan, precipitation occurs. To harden the nanoparticles with an average range of 373 ± 71 nm, glutaraldehyde is applied last [9][35][36]. This technique has several advantages over other methods, the most notable of which is its ability to create nanoparticles in a single step, in addition to its low costs, low use of electricity, and frequency [37][38]. Figure 3B shows an illustration of this strategy.
6. Nano Precipitation
The solvent displacement technique, commonly referred to as nanoprecipitation, has many benefits over other approaches. The principle of this fabrication method is known as the Marangoni effect. In the nanoprecipitation method, the nanoparticles are obtained from the colloidal suspension when the oil phase is slowly added to the aqueous phase under moderate stirring. The formation of the nanoparticles is instantaneous and needs only one step so it has the advantage of a rapid and straightforward operation. The key parameters in the fabrication procedure have a great influence on the nanoprecipitation method such as the organic phase injection rate, aqueous phase agitation rate, and the oil phase/aqueous phase ratio [39]. Particle sizes with very narrow distribution can be synthesised because of the absence of shearing stress. This method is used mostly for hydrophobic drug entrapment but is sometimes employed to incorporate hydrophilic drugs. Polymers and drugs are dissolved in a water-miscible organic solvent, for example, acetone or methanol. The solution is then added to an aqueous solution that contains a surfactant in a drop-wise manner. Through rapid solvent diffusion, the nanoparticles are formed immediately. Afterwards, the solvents are removed under reduced pressure. A diffusing phase is created by dissolving chitosan in a solvent system and injecting it into the dispersion phase, i.e., methanol, through the membrane using a peristaltic pump at a constant flow rate of 0.8 mL/min. Tween 80 is mixed into the dispersion phase to obtain nanoparticles [6]. The nanoprecipitation approach can also generate nanoparticles with sizes ranging from 50 to 300 nm, which is advantageous because smaller particle sizes generate more areas of contact. This property is critical for its use in adsorption and desorption systems [40][41]. This method can generate particles as small as 170 nm, which increases the number of applications as well as its efficiency. Figure 4A shows a schematic representation of this method.
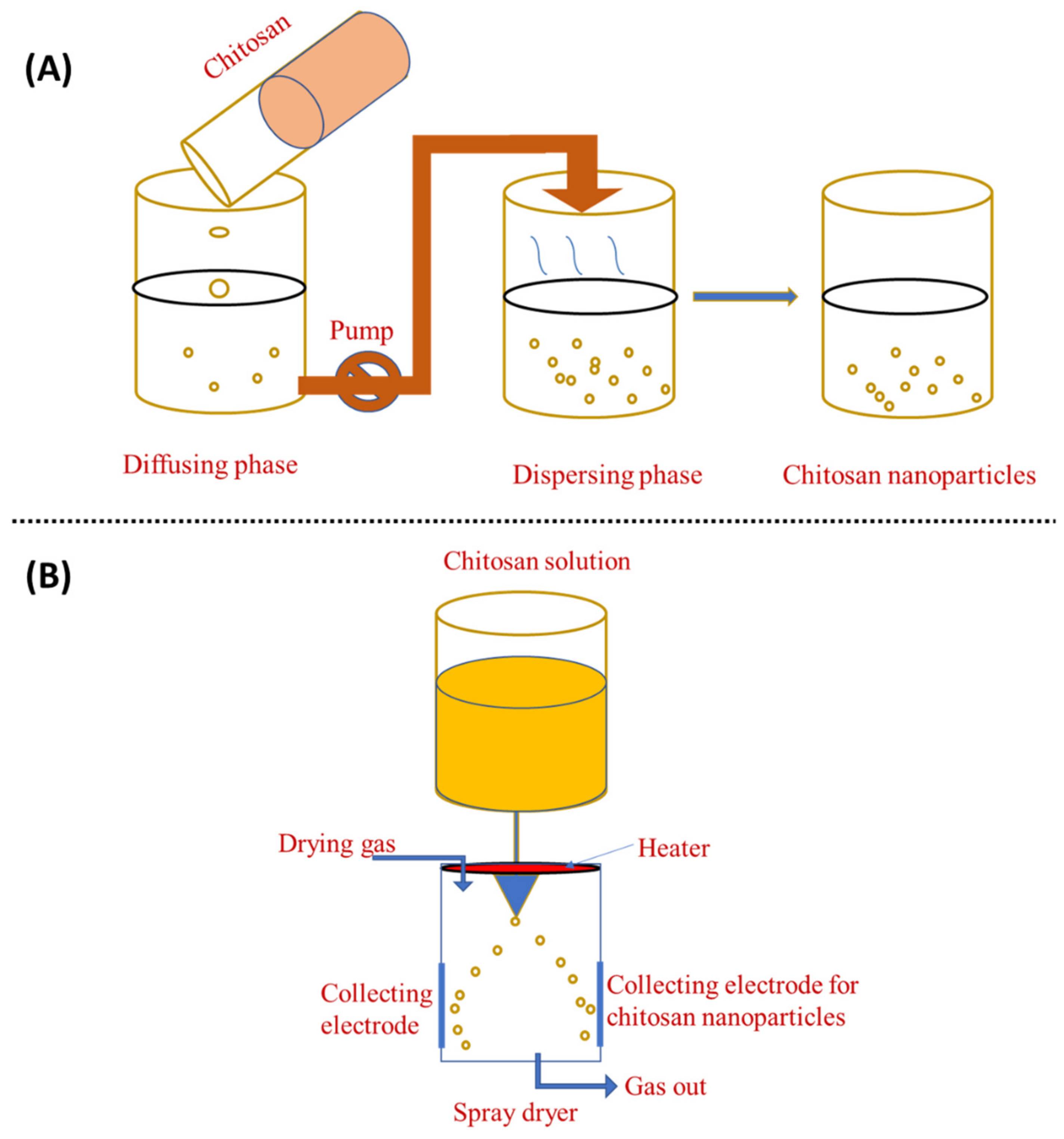
Figure 4. Preparation of chitosan nanoparticles by (A) nanoprecipitation method and (B) spray-drying method.
7. Spray-Drying
Spray-drying is another method for producing ChNP, as shown in Figure 4B. In this process, a nano spray dryer is used. Chitosan is solubilised with glacial acetic acid in water, which is then stored overnight. The solution is then atomised, which helps to generate droplets using an atomiser. The liquid phase is then evaporated by mixing these droplets with a drying gas, resulting in the formation of ChNP. Generally, the spray-drying nozzle size is 4.0, 5.5, or 7.0 μm and the flow rate is 2 mL/min; the drying gas flow is 1.3 m3/min, the inlet temperature 120 °C, and the outlet temperature 80 °C [42]. In the spray-drying process, the features and manufacturing yield of ChNP are influenced by the original feed, as well as the operating parameters, such as flow rate, nozzle size, and inlet and outlet temperatures [22]. In the pharmaceutical industry, spray-drying is frequently used to create the microencapsulation of antibiotics such as ampicillin, amoxicillin, vancomycin, etc [22]. Spray-drying is a simple, one-stage, continuous process that is only sluggishly influenced by the solubility of the drug and polymer. It can also be employed with pharmaceuticals that are heat-resistant, heat-sensitive, water-soluble, or water-insoluble and for hydrophilic or hydrophobic polymers [42]. Spray-drying is a simple, one-step approach that is protein-friendly for protein-loaded ChNP [43]. Ozturk et al. [44] used spray-drying to create ChNP comprising dexketoprofen and evaluated them for anti-inflammatory activity. ChNP loaded with dexketoprofen trometamol appear to be a potential oral prolonged-release medication delivery strategy with low dosages and good efficiency.
This entry is adapted from the peer-reviewed paper 10.3390/ma15196521
References
- Fonte, P.; Andrade, J.C.; Seabra, V.; Sarmento, B. Chitosan-based nanoparticles as delivery systems of therapeutic proteins. In Therapeutic Proteins; Springer: Berlin/Heidelberg, Germany, 2012; pp. 471–487.
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014.
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99.
- Shiraishi, S.; Imai, T.; Otagiri, M. Controlled release of indomethacin by chitosan-polyelectrolyte complex: Optimization and in vivo/in vitro evaluation. J. Control. Release 1993, 25, 217–225.
- Ohya, Y.; Takei, T.; Kobayashi, H.; Ouchi, T. Release behaviour of 5-fluorouracil from chitosan-gel microspheres immobilizing 5-fluorouracil derivative coated with polysaccharides and their cell specific recognition. J. Microencapsul. 1993, 10, 1–9.
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300.
- Tiyaboonchai, W. Chitosan nanoparticles: A promising system for drug delivery. Naresuan Univ. J. Sci. Technol. 2013, 11, 51–66.
- Sailaja, A.K.; Amareshwar, P.; Chakravarty, P. Different techniques used for the preparation of nanoparticles using natural polymers and their application. Int. J. Pharm. Pharm. Sci. 2011, 3, 45–50.
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28.
- Kafshgari, M.H.; Khorram, M.; Khodadoost, M.; Khavari, S. Reinforcement of chitosan nanoparticles obtained by an ionic cross-linking process. Iran. Polym. J. 2011, 20, 445–456.
- Rayment, P.; Butler, M.F. Investigation of ionically crosslinked chitosan and chitosan–bovine serum albumin beads for novel gastrointestinal functionality. J. Appl. Polym. Sci. 2008, 108, 2876–2885.
- Shi, L.; Chen, M.; Xinf, L.; Guo, X.; Zhao, L. Chitosan nanoparticles as drug delivery carriers for biomedical engineering. J. Chem. Soc. Pak. 2011, 33, 929–934.
- Shu, X.; Zhu, K. Chitosan/gelatin microspheres prepared by modified emulsification and ionotropic gelation. J. Microencapsul. 2001, 18, 237–245.
- Calvo, P.; Remuñan-López, C.; Vila-Jato, J.L.; Alonso, M.J. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm. Res. 1997, 14, 1431–1436.
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132.
- Patil, J.; Kamalapur, M.; Marapur, S.; Kadam, D. Ionotropic gelation and polyelectrolyte complexation: The novel techniques to design hydrogel particulate sustained, modulated drug delivery system: A review. Dig. J. Nanomater. Biostruct. 2010, 5, 241–248.
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27.
- Liu, H.; Gao, C. Preparation and properties of ionically cross-linked chitosan nanoparticles. Polym. Adv. Technol. 2009, 20, 613–619.
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-u. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials 2020, 10, 1970.
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73.
- Jonassen, H.; Kjøniksen, A.-L.; Hiorth, M. Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromolecules 2012, 13, 3747–3756.
- Ilium, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331.
- Mottaghitalab, F.; Farokhi, M.; Shokrgozar, M.A.; Atyabi, F.; Hosseinkhani, H. Silk fibroin nanoparticle as a novel drug delivery system. J. Control. Release 2015, 206, 161–176.
- Tokumitsu, H.; Ichikawa, H.; Fukumori, Y. Chitosan-gadopentetic acid complex nanoparticles for gadolinium neutron-capture therapy of cancer: Preparation by novel emulsion-droplet coalescence technique and characterization. Pharm. Res. 1999, 16, 1830–1835.
- El-Shabouri, M.H. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 2002, 249, 101–108.
- Niwa, T.; Takeuchi, H.; Hino, T.; Kunou, N.; Kawashima, Y. Preparations of biodegradable nanospheres of water-soluble and insoluble drugs with D, L-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method, and the drug release behavior. J. Control. Release 1993, 25, 89–98.
- Karnchanajindanun, J.; Srisa-ard, M.; Baimark, Y. Genipin-cross-linked chitosan microspheres prepared by a water-in-oil emulsion solvent diffusion method for protein delivery. Carbohydr. Polym. 2011, 85, 674–680.
- Perera, U.; Rajapakse, N. Chitosan nanoparticles: Preparation, characterization, and applications. In Seafood Processing By-Products; Springer: Berlin/Heidelberg, Germany, 2014; Volume 7, pp. 371–387.
- Brunel, F.; Véron, L.; David, L.; Domard, A.; Delair, T. A novel synthesis of chitosan nanoparticles in reverse emulsion. Langmuir 2008, 24, 11370–11377.
- Pileni, M. Reverse micelles used as templates: A new understanding in nanocrystal growth. J. Exp. Nanosci. 2006, 1, 13–27.
- Kafshgari, M.H.; Khorram, M.; Mansouri, M.; Samimi, A.; Osfouri, S. Preparation of alginate and chitosan nanoparticles using a new reverse micellar system. Iran. Polym. J. 2012, 21, 99–107.
- Zhao, L.-M.; Shi, L.-E.; Zhang, Z.-L.; Chen, J.-M.; Shi, D.-D.; Yang, J.; Tang, Z.-X. Preparation and application of chitosan nanoparticles and nanofibers. Braz. J. Chem. Eng. 2011, 28, 353–362.
- Liang, Z.; Yang, Z.; Yuan, H.; Wang, C.; Qi, J.; Liu, K.; Cao, R.; Zheng, H. "A –organic framework nanocomposite for pH-triggered anticancer drug delivery". Dalton Trans. 2018, 47, 10223–10228.
- Golińska, P. Biopolymer-based nanofilms: Utility and toxicity. In Biopolymer-Based Nano Films; Elsevier: Amsterdam, The Netherlands, 2021; pp. 353–385.
- Yu, L.; Wang, H.; Zhang, Y.; Zhang, B.; Liu, J. Recent advances in halloysite nanotube derived composites for water treatment. Environ. Sci. Nano 2016, 3, 28–44.
- Gonzalez-Melo, C.; Garcia-Brand, A.J.; Quezada, V.; Reyes, L.H.; Muñoz-Camargo, C.; Cruz, J.C. Highly efficient synthesis of type B gelatin and low molecular weight chitosan nanoparticles: Potential applications as bioactive molecule carriers and cell-penetrating agents. Polymers 2021, 13, 4078.
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091.
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics 2020, 12, 604.
- Luque-Alcaraz, A.G.; Lizardi-Mendoza, J.; Goycoolea, F.; Higuera-Ciapara, I.; Argüelles-Monal, W. Preparation of chitosan nanoparticles by nanoprecipitation and their ability as a drug nanocarrier. RSC Adv. 2016, 6, 59250–59256.
- Barreras-Urbina, C.G.; Ramírez-Wong, B.; López-Ahumada, G.A.; Burruel-Ibarra, S.E.; Martínez-Cruz, O.; Tapia-Hernández, J.A.; Rodriguez Felix, F. Nano-and micro-particles by nanoprecipitation: Possible application in the food and agricultural industries. Int. J. Food Prop. 2016, 19, 1912–1923.
- Khan, I.U.; Serra, C.A.; Anton, N.; Vandamme, T.F. Production of nanoparticle drug delivery systems with microfluidics tools. Expert Opin. Drug Deliv. 2015, 12, 547–562.
- Ngan, L.T.K.; Wang, S.-L.; Hiep, Đ.M.; Luong, P.M.; Vui, N.T.; Đinh, T.M.; Dzung, N.A. Preparation of chitosan nanoparticles by spray drying, and their antibacterial activity. Res. Chem. Intermed. 2014, 40, 2165–2175.
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652.
- Öztürk, A.A.; Kıyan, H.T. Treatment of oxidative stress-induced pain and inflammation with dexketoprofen trometamol loaded different molecular weight chitosan nanoparticles: Formulation, characterization and anti-inflammatory activity by using in vivo HET-CAM assay. Microvasc. Res. 2020, 128, 103961.
This entry is offline, you can click here to edit this entry!
