1. Introduction
The Neurovascular Unit (NVU) is a novel and transforming concept formalized in 2001 by the Stroke Progress Review Group of the National Institute of Neurological Disorders and Stroke [
1]. As implied by its name, the NVU is constituted by elements of the nervous and vascular systems. The neural elements consist of astrocytes, pericytes, microglia, peripheral immune cells, and neurons, while the vascular components include Endothelial Cells (ECs), Vascular Smooth Muscle Cells (VSMCs), and pericytes. Together, these cellular networks are responsible for the cellular interplay from neuron-to-vessel communication, Neurovascular Coupling (NVC), vessel-to-neuron signaling, vasculo-neuronal coupling to maintain brain homeostasis, and responding to inflammation and disease. The unique structure of the NVU along with transmembrane proteins forms a barrier that regulates the movement of molecules between the blood and brain, called the blood–brain barrier (BBB) (
Figure 1). Many neurological diseases are associated with the breakdown of this interplay, resulting in increased permeability of the BBB and neuronal dysfunction [
2,
3]. In the event of a stroke, the NVU plays a pivotal role in the progression of stroke injury and remains the main target of neuroprotective therapy.
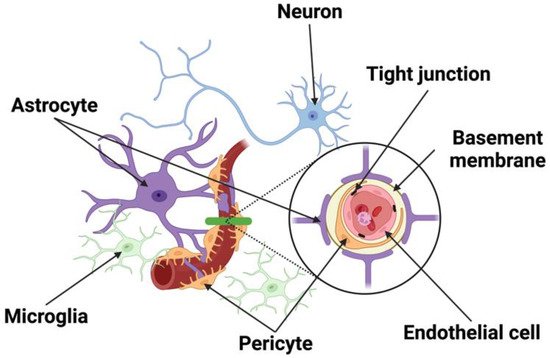
Figure 1. Schematic of the NVU. The NVU is comprised of neurons, vascular cells (endothelial cells, pericytes), basement membrane, and glia (astrocytes, microglia). Neurons make distinct connections with blood vessel and other cells of the NVU. Endothelial cells forming the blood vessels are encased by a basal lamina/basement membrane and are bound by tight junction proteins. Located in the brain parenchyma, astrocytes make contact with both pericytes and endothelial cells at the capillary wall, while pericytes are situated between the end feet of astrocytes and endothelial cells.
2. The Structural Components of the Neurovascular Unit (NVU)
2.1. Neurons
Different types of neurons including noradrenergic [
4,
5], serotonergic [
6], cholinergic [
7,
8], and GABAergic [
9] neurons have been shown to make distinct connections with other cells of the NVU, allowing regional brain activity to be metabolically coupled to blood flow [
10]. Evidence shows that BBB opening may be a selective compensatory event rather than a simple anatomical disruption, implicating that interaction between neurons and the brain microvasculature may regulate both blood flow and BBB permeability.
2.2. Endothelial Cells
ECs line blood vessels in the brain and are joined with one another by specialized Tight Junctions (TJs) and Gap Junction (GJ) proteins consisting of various molecular components [
11,
12,
13,
14]. These TJs form the BBB, a physical barrier between blood and brain parenchyma [
15], and regulate the permeability of the EC layer that keeps unwanted molecules such as toxins from entering the brain. This organization supports the current view that the BBB is not just a physical “barrier”, but a dynamic and metabolic interface [
16,
17].
Capillaries make up the microcirculation and are divided into three types: continuous, fenestrated, and sinusoidal. Brain capillaries belonging to the continuous type have no perforations on the vessel wall, allowing only small molecules to pass through. In contrast, fenestrated capillaries in the kidneys and GI tract along with sinusoidal capillaries in the lymphoid organs are leakier and have small and large pores, respectively. They allow for the passage of large molecules and even cells compared with the brain capillaries. By directly isolating tissue-specific mRNAs using the RiboTag transgenic mouse model [
18], a recent study compared the transcriptome profiles of ECs from the brain, heart, and lung using RNA sequencing. The authors found that each EC type had a distinct genetic signature under normal conditions and an organ-specific response to lipopolysaccharide. Most surprisingly, ECs turned on genes that were expressed by the surrounding tissue upon stimulation, indicating organ-specific endothelial plasticity and adaptation [
19]. Specifically in the brain, genes involved in processes akin to neuronal function such as synapse organization, neurotransmitter transport, axon development, and ion transport regulation are also enriched in the brain ECs [
19].
Endothelial cells also interact with other components in the brain, such as pericytes, astrocytes, neurons, and the ECM to maintain the normal function of NVU by forming the BBB [
16,
20]. The interplay among partners in the NVU and the integrity of the BBB is key to brain homeostasis. The interaction between capillaries and neuronal components in neurovascular coupling regulates CBF through the detection of changes in shear stress of the endothelial wall and the supply of oxygen and necessary nutrients in the brain tissue. BBB breakdown leads to an influx of toxic molecules towards the brain, causing neuronal injury and neurodegenerative changes. While the NVU and BBB are critical for the maintenance of Central Nervous System (CNS) homeostasis, they could also interfere with the delivery of therapeutic drugs into the CNS via systemic administration for the treatment of neurological diseases. Further research and clinical trials are needed to successfully deliver necessary drugs to the CNS without causing inadvertent permeability of the BBB.
2.3. Pericytes
Pericytes surround cerebral vessels and are intimately in contact with the ECs embedded in the vascular basement membrane (BM) through gap junctional complexes around pericyte somata [
21,
22], called peg-socket contacts. Like endothelial cells, pericytes are attached to extracellular matrix proteins of the BM by different integrins that control junctional complex protein expression [
23], affecting the functionality of the BBB by controlling both the structure of TJs and the rate of vesicular trafficking. They are also involved in various vascular functions such as BBB formation andmaintenance, angiogenesis, vessel maturation, regulation of blood flow, and immune cell trafficking [
24,
25,
26,
27]. Pericytes can inhibit the expression of genes that promote vessel permeability. Pericytes control neuroinflammation by reducing leukocyte trafficking in the regions of blood vessels they cover [
24]. A study using pericyte-deficient mice showed a correlation between the reduction of tight junction and adherens junction proteins and the resulting increase in paracellular leakage and eventually BBB breakdown [
25]. The resulting permeability leads to an influx of neurotoxic macromolecules, water, and larger molecules that are normally incapable via increased endothelial transcytosis [
26,
27], and a reduction in capillary blood flow due to microvascular degeneration [
28].
Capillary vessels dilate to accommodate oxygen demand in the brain in response to physiological stimulation such as hypercapnia or sensory stimulation. Earlier studies implied that pericytes can contract or relax, resulting in changes in the capillary diameter under various physiological and pathological conditions [
29,
30,
31,
32]. To further distinguish which cells in the vessel zones respond to stimulation, a study using optogenetics found that it was SMCs rather than pericytes that contracted when mural cells expressing ChR2 were stimulated [
30]. However, another study found that pharmacological inhibitor of myosin contraction signaling along with optical ablation of capillary pericytes resulted in consistent dilation of regions lacking pericyte contact, leading to aberrantly increased flux of blood flow in the uncovered capillary vessels [
21,
33]. A recent scRNAseq study suggests that pericytes do have the molecular machinery to regulate vessel diameter since capillary pericytes express receptors for vasoactive mediators including L-type voltage-gated calcium channels and those involved in smooth muscle cell actomyosin contraction [
34], providing pivotal support for the modulatory role of pericytes in controlling capillary diameters and homogenizing blood flow to facilitate oxygen extraction, particularly during functional hyperemia. Apart from their modulatory role, pericytes seem multipotent and are known to differentiate into both neural and vascular lineage cells after brain ischemia [
35,
36].
The same group of investigators who found pericytes drive capillary vessel dilation reported that pericyte loss and capillary dilation caused by focal ablation of pericytes with laser was exacerbated in the aged brain, resulting in increased flow heterogeneity in capillary networks. Although the remodeling of neighboring pericytes restored endothelial coverage and vascular tone within days, this process was slower in the aged brain and led to persistent capillary dilation [
37]. This suggests that pericytes do communicate with one another and work together to maintain neurovascular coupling. In support of this notion, one study showed that neighboring pericytes in the mouse retina can communicate with each other in response to light stimulation by forming interpericyte tunneling nanotubes that become a functional network with an open-ended proximal side and a closed-ended end-foot that connects with distal pericyte processes via gap junctions, serving as a conduit for intercellular Ca
2+ waves between pericytes [
38].
2.4. Astrocytes
Linking the vascular and neural systems are perivascular astrocytes, whose astrocytic processes almost completely encapsulate the abluminal EC surface of brain vessels. Astrocytes play a role in the development of junctional complexes and physically link neighboring neurons with blood vessels [
39,
40,
41], allowing them to detect changes in the neuronal microenvironment and adjust the microvasculature appropriately [
42,
43]. Perivascular astrocytes increase the tightness of TJs [
44], promote the expression of endothelial transporters [
45], and enzymes associated with the endothelial barrier [
40]. Through the secretion of bioactive substances, astrocytes provide physical support and strengthen the BBB, leading to TJ modulation. Gap junctions present between astrocytic end feet and vessel walls mediate intercellular communication and solute movement between them, such as water and ion exchange across the brain microvascular endothelium [
40,
46]. Water channel aquaporin 4 is noticeably expressed on astrocytic end feet and regulates water movement between the blood and brain [
47]. Astrocytes are known for their roles in responding to CNS injury by taking up excess glutamate from the extracellular space and converting it to glutamine [
48] to aid in the generation of new neurons, remodeling synapses, and recycling neurotransmitters [
24]. Critical to neuronal survival and repair, a large part of this function is mediated by gap junction proteins that connect astrocyte networks into a functional syncytium [
13,
49,
50,
51]. Through the secretion of proinflammatory (Interleukin (IL) IL-6 and IL-1β), anti-inflammatory cytokines (IL-10), and chemokines (CCL2, CXCL1, CXCL10, and CXCL12), astrocytes can control microglia differentiation and macrophage activation [
52,
53,
54]. These cytokines lead to hyperplasia of astrocytes, which results in the expression of inflammatory factors that can lead to reactive gliosis and scar formation. Astrocytes can directly restrict the entry of peripheral immune cells through the BBB. They are also the source of MMPs, a family of extracellular proteinases that degrade TJs and the ECM after ischemia, leading to the detachment of astrocytic end feet [
55].
2.5. Microglia and Macrophages
Microglia are resident CNS macrophages that originate from the mesoderm during embryonic development and are widely distributed within the CNS. However, the basal ganglia and cerebellum have a higher abundance of microglia than the cerebral cortex [
56]. They migrate into the brain and are termed “resting microglia” due to their low phagocytotic properties [
57]. These cells communicate with endothelium to help regulate the BBB. As the primary immune cells that account for ~5–15% of all cells in the human brain, microglia can undergo morphological changes that allow them to phagocytose and produce pro-inflammatory cytokines IL-1 and IL-6, and enhance the expression of Intercellular Adhesion Molecule-1, P-selectin, and E-selectin [
55,
58]. As a result, the accumulation, migration, and adherence of leukocytes across endothelium allow them to mediate inflammatory cascades that further exaggerate the level of infarction.
During disease/trauma microglia become activated, and the extent of their activation is correlated to the severity and type of brain injury [
59]. Activation of microglia is associated with dysfunction of the BBB via changes in TJ protein expression and increased BBB permeability [
2]. High levels of neurotoxic mediators such as nitric oxide, peroxide, inflammatory cytokines (i.e., Tumor Necrosis Factor-α (TNF-α)), and proteases, as well as complement components [
59,
60], are produced, ultimately leading to cell injury in the CNS and neuronal cell death. Microglia are adept at sensing any small disturbance in the BBB [
61,
62] and maintaining BBB integrity during inflammation.
2.6. Junctional Complexes
ECs form the inner lining of blood vessels, creating a barrier between vessels and tissues. Lateral spaces between adjacent ECs called TJs, or Zona Occludins (ZO), and their proteins control the low paracellular permeability and high electrical resistance of the BBB [
57]. Common transmembrane TJ proteins include Claudins (primarily Claudin-5) and occludin, which are phosphoproteins with four transmembrane domains that span the intracellular cleft, binding to proteins on adjacent ECs. Claudins and occludin are associated with cytoskeletal signaling proteins such as ZO-1 and ZO-2 and link TJs to the primary cytoskeleton proteins like actin for the maintenance of structural and functional integrity of the endothelium [
63]. Junctional adhesion molecules are another family of transmembrane proteins that have a single transmembrane domain and are located at the borders of endothelial cells. These molecules are involved in cell-to-cell adhesion and leukocyte transmigration across the BBB [
64]. The regulation of polar solutes and macromolecules across the barrier prevents the passage of unwanted and potentially damaging material such as peptides and proteins between the blood and brain. Through mechanoreceptor properties, ECs can respond and adjust vascular resistance via vasodilation and constriction to compensate for alterations of perfusion pressure and maintain a relatively constant CBF and microvascular pressure that contribute to cerebral autoregulation [
65]. Akin to TJs, adherens junctions are protein complexes that occur at cell-cell junctions in endothelial cells. Unlike TJs, AJs join and maintain the connection between actin filaments of the cytoskeleton of neighboring cells. Transmembrane proteins called cadherins are a group of proteins that bind with other cadherins on adjacent ECs to help ECs stick together and regulate the intracellular signaling pathways that control gene transcription [
66]. Additionally, gap junctions are intercellular channels that allow ions and molecules to pass through resulting in changes in membrane potential from one cell to another. Contrary to the extremely low permeability of tight junctions, gap junctions allow for the passage of certain molecules between cells. Consisting of connexin proteins, these structures allow for rapid propagation of action potentials along with the slow diffusion of nonorganic ions, secondary messengers, and other small water-soluble molecules [
58]. They also transmit chemical signals and metabolites between cells, aiding in the function of vascular, neuronal and glial tissue [
58]. Degradation of gap junctions contributes to the release of inflammatory mediators and disrupts the homeostasis of the CNS environment as exemplified under condition of ischemic stroke. These intercellular junctions are important for providing efficient and selective barriers against undesirable environmental conditions, providing the structural integrity of the cells that make up this barrier, and the overall homeostasis of the brain.
2.7. Basement Membrane
The basement membrane (BM) or ECM is an amorphous structure located on the abluminal side of endothelial cells or basal side of epithelial cells [
67,
68], and is comprised of multiple components such as laminins, collagen, nidogen, and heparan sulfate proteoglycans [
67,
68,
69,
70], forming a close contact with the endothelium. The BM plays a crucial role in maintaining vascular integrity and providing a rigid support to vessels and surrounding cells [
70] by surrounding the capillaries and separating them from neighboring astrocytes and pericytes [
71]. The ECM of the basement membrane limits the transmigration of red blood cells during hemorrhage and leukocytes during inflammation. Dysfunction and degradation of the BM are associated with several neurological disorders and increased barrier breakdown and edema [
72,
73]. Interestingly, endothelial cells, astrocytes, and pericytes are known to synthesize and deposit specific laminin isoforms in the BM that modulate BBB function [
74,
75].
This entry is adapted from the peer-reviewed paper 10.3390/cells11182823