Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Obstetrics & Gynaecology
Gestational diabetes mellitus (GDM), a glucose intolerance developing or first recognized during pregnancy, leads to a series of short- and long-term maternal and fetal complications, somehow related to placenta structural and functional changes. The placenta has a key role in correct fetal development. A series of factors are related to the correct functioning of the placenta as maternal and fetal blood flow, expression and function of receptors and transporters, and appropriate nutrients.
- gestational diabetes
- placenta
- mass spectrometry
1. Introduction
As an introduction we consider it essential to describe the biological and medical aspects of the placental pathophysiologies. The placenta has a key role in correct fetal development. A series of factors are related to the correct functioning of the placenta as maternal and fetal blood flow, expression and function of receptors and transporters, and appropriate nutrients. It is well known that placenta in diabetes undergo a series of structural and functional changes due to the abnormal maternal milieu determined by elevated levels of glucose ad insulin [1]. Gestational diabetes mellitus (GDM), a glucose intolerance developing (or first recognized) during pregnancy that is not an overt diabetes [2], has been increasing over the last decade. It accounts for 12–18% of all pregnancies and is due mainly to the increased frequency of obesity [2,3]. This condition, if not properly diagnosed and treated, determines a series of short- and long-term maternal and fetal complications such as preeclampsia, cesarean delivery, birth trauma, macrosomia, neonatal hypoglycemia and hyperbilirubinemia [3,4,5]. Furthermore, women affected by GDM and their children are at high risk of developing cardiometabolic diseases (including type 2 diabetes, obesity, hyperlipemia, metabolic syndrome, hypertension, and cardiovascular disease) later in life [4,5]. GDM develops when beta cell insulin secretion is unable to compensate for the physiological pregnancy-induced insulin resistance, and/or in conjunction with an impaired beta cell function [6,7]. In this context, as recently evidenced, genetic, epigenetic and environmental factors contribute in determining insulin resistance and beta-cell disfunction, and consequently GDM development. In addition, the adverse intrauterine environment in patients with GDM could also have a negative impact on the establishment of the epigenomes of the offspring [6,7,8]. Furthermore, GDM is associated with altered concentrations of nutrients, inflammatory cytokines which can contribute to placenta modifications. This includes changes in the surface area and volume, as well as histological changes as an increased volume of the intervillous space and terminal villi, the number of syncytiotrophoblast, fibrinoid and glycogen deposits. These modifications may result in functional changes of the placenta, with consequent impairment of fetal development [1]. Recently, the new omics methodologies have been retained, as they are useful for the identification of new pathways and processes that could be affected in placenta modified by diabetes [9]. In this context, proteomics, based on the structural identification and quantitative evaluation of the proteins present in a cell, tissue or organism in a well-defined moment, and related to pathological conditions, has been proved to be a highly valid approach, together with metabolomics, genomic and metallomics [10]. It must be considered that the changes of the various biochemical pathways characteristic of an organism originating by a specific disease, drug or physiological activity reflect in the identification of novel biomarkers, is useful in the management of the diseases in clinical practice [11]. The identification of modified proteins in placenta of GDM patients and their possible involvement in placenta and fetal development is of key importance in order to outline prevention strategies of maternal and fetal complications. In a review focused on proteomic studies on placenta samples and placenta-derived cells of normal pregnant women, Robinson et al. [12] concluded that proteomic analysis is a promising approach to obtain information that is difficult to obtain with traditional approaches in pathological conditions.
2. Placenta Protein Profile
In order to evaluate the results obtained in the study on the placenta protein profile, we considered it of interest to employ a general strategy based on four different aspects: (i) samples analyzed; (ii) instrumental approaches employed; (iii) obtained results; (iv) physiopathological meaning of the results. A preliminary investigation on the placenta protein profile [13] was based on pooled placenta omogenates from 20 healthy subjects and 20 GDM pregnant women.
The analytical strategy employed in the study is summarized in Figure 1. The biological samples under study were obtained by removing the placental cotyledon from the central region of the placenta to obtain villous tissue. Once thawed, 1 g of each placenta sample was washed with distilled water, dipped in ice-cold homogenization buffer and homogenized under ice-cooling with a blender. The homogenate was centrifuged twice at 15,000 rpm for 10 min. The supernatant, containing the hydrosoluble proteins, was recovered, collected and pooled in two groups: “control” and “diabetic”. The two pooling samples were directly analyzed by matrix-assisted laser desorption/ionization mass spectrometry (MALDI MS) [14], and the proteins detected were tentatively identified by comparison of their molecular weight with the Human Protein Reference Database, restricting the search to the species expressed in placenta tissue. At first sight this method seemed to lead to satisfactory results, with the detection in the m/z range 15,000–32,000 of: 39S ribosomal protein I.55, insulin–like peptide INSI.5, ergosterol biosynthetic protein 28, histone H3-like centromeric protein A, R-spondin-3 and mitocondrial dicarboxylate carrier. However, an in-depth analysis of these results showed the presence of relevant limits of this approach: the most abundant ionic species in the MALDI spectra were proved to be due to globins present in the maternal and fetal blood. Furthermore, an evaluation with MS/MS of the placenta samples omogenates subjected to trypsin digestion confirms this result. These findings focalize the attention that needs to be paid to avoid blood contamination of the placenta samples, and in the analysis of the data obtained from database comparison. The one-dimensional electrophoretic separation of the proteins present in the two pools was performed, followed by tryptic digestion of the proteins evidenced by the different bands (right part of Figure 1). With this accurate method, a large number of proteins in the mass range 15–220 kDa were found and, after digestion with trypsin, MALDI analysis made possible to identify a series of proteins (different from globins), with coverage values ranging from 34% to 78%. Densitometric analysis indicated a slightly higher optical density in the bands. This was due to a serine/arginine repetitive matrix protein 1 and Bcl2-associated transcriptor factor1 for the GDM pool with respect to the same bands in the control pool. These structural assignments were confirmed by MALDI MS/MS experiments, which in the case of samples from the diabetic pool, show also the presence of glyco-oxidized species, i.e., the products of non-ezymatic glycation and glycol-oxidation arising from the reaction of glucose with amino groups of proteins. It was noted that only minor quantitative differences were found between proteins for GDM and normal pregnancies; these data can be explained by the fact that the GDM women evaluated were in good metabolic control, showing only slightly elevated HbA1c values with respect to normal pregnant women. It could be emphasized that the analysis of a pool sample exhibits some limitations, giving a general view of the possible differences but lacking the aspects related to single subject.
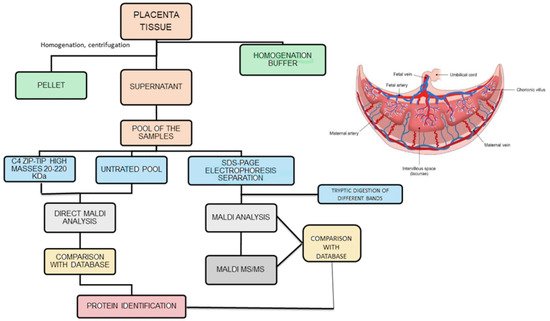
Figure 1. Analytical strategies employed to analyze placenta samples (homogenate was centrifuged twice at 15,000 rpm).
To evaluate the role of the placenta sample treatments on the achievable results, it was considered of interest to conduct an investigation on the placenta protein profile. This was carried out by matrix-assisted laser desorption/ionization ion imaging [15] experiments, performed by direct laser irradiation of the maternal and fetal sides of the placenta tissue [16]. To investigate the possible GDM-induced changes, the results obtained for five placenta samples from GDM patients were compared with those from five placenta samples of healthy pregnant women. This investigation required an in-depth evaluation of the sample treatments for the MALDI ion imaging operative conditions: four different operative protocols were tested and the stainless–steel sieve method [17] for matrix deposition proved to be particularly effective. However, as already observed in the MALDI spectra of placenta omogenates, the most abundant peaks obtained by direct irradiation of the placenta tissue are due to blood globins, even after the sequential washing procedure (up to five). This behavior was also observed by Wang et al. [18] in screening multi-protein complexes in placenta: heterooligomeric multi-protein complexes (including mitochondrial respiratory chain, integrin, proteasome, histone, and heat shock protein complexes) were identified. These results were obtained by utilizing an electrophoresis method (BN SDS-PAGE) coupled with a mass spectrometry approach (LC-MS/MS), i.e., in experimental conditions leading to results more specific with respect to those achievable by MALDI. Attention was then focused on the species detected in the m/z range 20,000–47,000. The average MALDI ion imaging spectra, obtained for two placenta samples taken as an example, evidenced differences in the absolute abundances of the ionic species at m/z 30.335, m/z 31,235 and m/z 32,000, due to NADH dehydrogenase iron-sulfur protein 3, mitochondrial, transcription cofactor vestigial-like protein 4, voltage dependent anion-selective channel protein 2 respectively. An interesting result was observed for the ion at m/z 31,325 (transcription cofactor vestigial-like protein 4): it is the most abundant species only in the case of the maternal placenta side of the GDM subject, indicating that changes in the placenta protein profile in GDM patients are present mainly in the maternal side of the placenta, and that the placenta action seems to inhibit these changes on the fetal side. However, the low number of patients under study must be stressed, indicating the necessity of a large number of healthy and GDM subjects. When these data were evaluated in the 20 placenta samples under investigation, the differences above discussed are not so evident. The ion intensity from the average spectra of the different samples show that clear differences are present for the ions at m/z 30,335 and 31,235 between the maternal and fetal sides, both for healthy and the GDM pregnant women. It was noted that many of the detected proteins are present at the mitochondrial These preliminary results seemed to be very promising as evidence of differences at the protein level between maternal and fetal sides of the placenta. However, in our opinion, in order to confirm these findings and to evaluate their physiopathological meaning, further work is required on a larger number of placenta samples. Only after this in-depth further analyses it would be possible to evaluate which placental side should be used in future experiments.
The above-described approaches exhibit severe limitations. Consequently, it was considered interesting to utilize the classical and more specific approaches employed in proteomics, i.e., 2D gel-based electrophoresis followed by nanoLC-MS/MS or MALDI-MS/MS and MSE (gel free) analysis of the different spots. The data obtained by 2D gel-based and gel-free proteomic approaches on human placental tissue of GDM patients were then compared [19]. The gel-based approach highlights, in the case of GDM samples, 13 over-expressed proteins and 16 under-expressed proteins. This underlines that, while nanoLC-MS/MS shows that electrophoresis spots contain more than one protein, MALDI-MS/MS leads to the identification of only one protein per spot, indicating the highest effectiveness of the former analytical approach. The gel-free approach was successively applied to placenta tissues, and the enzymatic digestion products of the whole placental tissue were analyzed by a label-free LC-MSE method. This method allows not only the identification but also the quantification of proteins. The results obtained can be ascribed to a reproducible chromatographic separation followed by the MS/MS analysis, performed by alternating acquisition of product ion spectra at lower and higher collision energies. This approach led to the detection of 159 proteins, of which 10 over-expressed in GDM placental tissue and 9 under expressed compared to normal placenta: Periostin, Ig gamma-2 chain C region, CSH, Moesin, Heat-shock-related 70 kDa protein 2, triosephospate isomerase, Protein disulfide-isomerase, Galectin-1, Vimentin, 14-3-3 protein beta/alpha. Some of these proteins were identified also in the analysis performed with the gel-based approach.
Differently to what was performed in previous investigations, based on the pooling of placenta samples, in a further study [20] each placenta sample obtained from healthy (12) and GDM (13) subjects was analyzed individually by means of the label-free LC-MSE method. This approach allowed us to identify 3776 peptides, corresponding to 160 proteins; statistical analysis allowed us to highlight higher levels of galectin 1 and collagen alpha-1 chain in the case of GDM samples, while heat shock protein 1A/1B was less abundant in the GDM placental tissue. Galectin-1 is a carbohydrate-binding protein with affinity for beta-galactosides, which in pregnancy has been implicated in regulating processes associated with adaptation to pregnancy and in mechanisms involved in angiogenesis, trophoblast invasion, and syncytium formation. Its dysregulation has been associated with adverse pregnancy outcomes as spontaneous abortion, preeclampsia, and HELLP syndrome. In a recent paper evaluating placental tissues with dual immunofluorescence, Blois et al. [21] showed that serum galectin-1 levels were reduced, while placental galectin-1 was overexpressed in GDM women. Furthermore, a slightly higher abundance of collagen alpha-1 chain was also found in placenta samples of GDM women.
It is noted that clear differences are present among the “diagnostic” proteins when considering the results of the study of Burlina et al. [20] and those identified in the previous studies based on pooled placenta samples. In the latter case, a general view of the protein map, due to proteins present in different samples, is obtained. On the contrary, the findings on specific placenta samples indicate that the placental proteome is weakly affected by GDM. This is different to what was observed for metabolome, which exhibits major changes in the presence of GDM. The placenta proteome appears to be scarcely modified by GDM, and this can be due to the good glycemic control of the patients evaluated; furthermore, the abnormalities put in evidence are the view of GDM at diagnosis, when patients are not treated with dietetic advices and/or insulin.
The modifications in placenta metabolism and uptake of nutrients can contribute to the long-term complications of neonate of GDM patients [29]. As an example, recent studies have evidenced that some adipokines secreted by adipose tissue (leptin, adiponectin) can impair the uptake of nutrient in the placenta [30]. Recently, attention has been focused on the role of exosomes, nanovesicles that contain proteins lipidis, mRNA, involved in the interorgan communication and whose composition changes in a series of pathological state as hypoxia and obesity [31]. In a study analyzing the exosomes derived by adipose tissue in diabetics with and without obesity, a differential expression of exosomes proteins has been reported [32]. Jayabalan et al. [33] in a very elegant study have analyzed, by mass spectrometry, the exosomes isolated from omental adipose tissue from NGT pregnant women (n = 65) and pregnant women with GDM (n = 82). The effect of exosomes on human placental cells has been determined using a Human Glucose Metabolism RT2 Profiler PCR array. The result of the study shows that the number of exosomes (vesicles/μg of tissue/24 h) was higher (1.7-fold) in pregnant women affected by GDM with respect to normal pregnant ones. Interestingly, a positive correlation was found between the babies’ birthweight and the number of exosomes. The pathway analysis of the exosomal proteins evidenced differential expression of the proteins targeting the sirtuin signaling pathway, oxidative phosphorylation, and mechanistic target of the rapamycin signaling pathway in GDM compared with NGT. Therefore, these data suggest that in GDM patients, the exosomes of the adipose tissue can modulate the genes involved in the metabolism of glucose of the placenta and the fetal growth.
In coclusion the data obtained until now, and described above, briefly described how the newest mass specrometric approaches are a valid tool to investigate the possible changes of placenta physiopathology.The results described above show that quite different results have been obtained in different studies and this can be due to 3 different aspects: 1) placenta tissue sampling, 2) instrumental approaches utilized for protein profile analysis, 3) protein identification. It is necessary that in the future these approaches will me more specific, utilizing accurate measurements utilizing ultrahigh resolution mass spectrometry. This will give more reliable results to be easily compared among different research groups, so to individuate the metaboli changes in GDM patients and to implement prevention strategies of maternal and fetal complications. However, to reach this aim, studies with a large number of patients and with a correct selection of the patients need to be performed.
.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10092272
This entry is offline, you can click here to edit this entry!
