Dendrimers are a special class of synthetic macromolecules, constituted of branches built, step by step, around a central multifunctional core. Each layer of branching points creates a new “generation”. Most of the properties of dendrimers depend on the type of their terminal functions. Dendrimers are often considered as 3-dimensional soft nanoparticles, in opposition to hard metal nanoparticles. Despite the fact that nature has favored branching structures at all levels, from galaxies to trees and to dendritic cells, examples of branching at the molecular level are extremely rare. One can cite glycogen, a branched polymer of glucose, and also some cases of branched lignin, but none of them have a precisely highly branched structure, as do dendrimers. Such unusual structure has generated a many expectations for dendrimers: a huge number of publications and patents exist in relation to medicine, including in relation to personalized medicine but have resulted in very poor clinical translation up to now. This entry focusses on some of the clinical trials carried out with dendrimers.
- dendrimers
- clinical trials
- phases 1, 2, and 3
- polylysine
- polyamidoamine
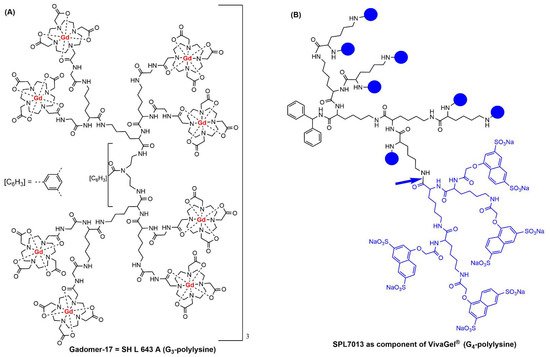
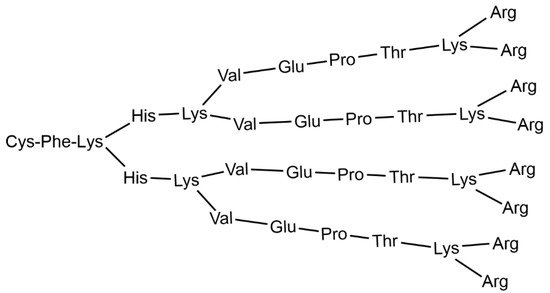
1. Clinical Trials with Dendrimers Based on Poly-L-Lysine
2. Clinical Trials with PAMAM Dendrimers
3. Clinical Trials with Other Types of Dendrimers
This entry is adapted from the peer-reviewed paper 10.3390/jpm12081334
References
- Denkewalter, R.G.; Kolc, J.; Lukasavage, W.J. Macromolecular Highly Branched Homogeneous Compound Based on Lysine Units. US Patent 4,289,872, 15 September 1981.
- Runge, V.M.; Heverhagen, J.T. Advocating the Development of Next-Generation High-Relaxivity Gadolinium Chelates for Clinical Magnetic Resonance. Investig. Radiol. 2018, 53, 381–389.
- Herborn, C.U.; Schmidt, M.; Bruder, O.; Nagel, E.; Shamsi, K.; Barkhausen, J. MR Coronary Angiography with SH L 643 A: Initial Experience in Patients with Coronary Artery Disease. Radiology 2004, 233, 567–573.
- Starpharma. Available online: https://starpharma.com (accessed on 2 August 2022).
- Holmes, W.R.; Maher, L.; Rosenthal, S.L. Attitudes of men in an Australian male tolerance study towards microbicide use. Sexual Health 2008, 5, 273–278.
- Chen, M.Y.; Millwood, I.Y.; Wand, H.; Poynten, M.; Law, M.; Kaldor, J.M.; Wesselingh, S.; Price, C.F.; Clark, L.J.; Paull, J.R.A.; et al. A Randomized Controlled Trial of the Safety of Candidate Microbicide SPL7013 Gel When Applied to the Penis. Jaids-J. Acquir. Immune Defic. Syndr. 2009, 50, 375–380.
- O’Loughlin, J.; Millwood, I.Y.; McDonald, H.M.; Price, C.F.; Kaldor, J.M.; Paull, J.R.A. Safety, Tolerability, and Pharmacoki-netics of SPL7013 Gel (VivaGel®): A Dose Ranging, Phase I Study. Sex. Transm. Dis. 2010, 37, 100–104.
- Cohen, C.R.; Brown, J.; Moscicki, A.B.; Bukusi, E.A.; Paull, J.R.A.; Price, C.F.; Shiboski, S. A Phase I Randomized Placebo Controlled Trial of the Safety of 3% SPL7013 Gel (VivaGel®) in Healthy Young Women Administered Twice Daily for 14 Days. PLoS ONE 2011, 6, e16258.
- Moscicki, A.B.; Kaul, R.; Ma, Y.; Scott, M.E.; Daud, I.I.; Bukusi, E.A.; Shiboski, S.; Rebbapragada, A.; Huibner, S.; Cohen, C.R. Measurement of Mucosal Biomarkers in a Phase 1 Trial of Intravaginal 3% StarPharma LTD 7013 Gel (VivaGel) to Assess Expanded Safety. J. Acquir. Immune Defic. Syndr. 2012, 59, 134–140.
- McGowan, I.; Gomez, K.; Bruder, K.; Febo, I.; Chen, B.A.; Richardson, B.A.; Husnik, M.; Livant, E.; Price, C.; Jacobson, C.; et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004). Aids 2011, 25, 1057–1064.
- Carballo-Dieguez, A.; Giguere, R.; Dolezal, C.; Chen, B.A.; Kahn, J.; Zimet, G.; Mabragana, M.; Leu, C.S.; McGowan, I. ‘‘Tell Juliana’’: Acceptability of the Candidate Microbicide VivaGel® and Two Placebo Gels Among Ethnically Diverse, Sexually Active Young Women Participating in a Phase 1 Microbicide Study. AIDS Behav. 2012, 16, 1761–1774.
- Price, C.F.; Tyssen, D.; Sonza, S.; Davie, A.; Evans, S.; Lewis, G.R.; Xia, S.; Spelman, T.; Hodsman, P.; Moench, T.R.; et al. SPL7013 Gel (VivaGel (R)) Retains Potent HIV-1 and HSV-2 Inhibitory Activity following Vaginal Administration in Hu-mans. PLoS ONE 2011, 6, e24095.
- Madan, R.P.; Dezzutti, C.S.; Rabe, L.; Hillier, S.L.; Marrazzo, J.; McGowan, I.; Richardson, B.A.; Herold, B.C.; Microbicide Trials Network, B.; Team, M.T.N.P. Soluble Immune Mediators and Vaginal Bacteria Impact Innate Genital Mucosal Anti-microbial Activity in Young Women. Am. J. Reprod. Immunol. 2015, 74, 323–332.
- Waldbaum, A.S.; Schwebke, J.R.; Paull, J.R.A.; Price, C.F.; Edmondson, S.R.; Castellarnau, A.; McCloud, P.; Kinghorn, G.R. A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose-ranging study of the efficacy and safety of Asto-drimer Gel for the treatment of bacterial vaginosis. PLoS ONE 2020, 15, e0232394.
- McCarthy, T.D.; Karellas, P.; Henderson, S.A.; Giannis, M.; O’Keefe, D.F.; Heery, G.; Paull, J.R.A.; Matthews, B.R.; Holan, G. Dendrimers as drugs: Discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2005, 2, 312–318.
- Chavoustie, S.E.; Carter, B.A.; Waldbaum, A.S.; Donders, G.G.G.; Peters, K.H.; Schwebke, J.R.; Paull, J.R.A.; Price, C.F.; Cas-tellarnau, A.; McCloud, P.; et al. Two phase 3, double-blind, placebo-controlled studies of the efficacy and safety of Asto-drimer 1% Gel for the treatment of bacterial vaginosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 13–18.
- Schwebke, J.R.; Carter, B.A.; Waldbaum, A.S.; Agnew, K.J.; Paull, J.R.A.; Price, C.F.; Castellarnau, A.; McCloud, P.; Kinghorn, G.R. A phase 3, randomized, controlled trial of Astodrimer 1% Gel for preventing recurrent bacterial vaginosis. Eur. J. Ob-stetrics Gynecol. Reprod. Biol. X 2021, 10, 100121.
- VIRALEZE. Available online: https://www.starpharma.com/viraleze/spl7013 (accessed on 2 August 2022).
- Castellarnau, A.; Heery, G.P.; Seta, A.; Luscombe, C.A.; Kinghorn, G.R.; Button, P.; McCloud, P.; Paull, J.R.A, Astodrimer sodium antiviral nasal spray for reducing respiratory infections is safe and well tolerated in a randomized controlled trial. Sci. Rep. 2022, 12, 10210.
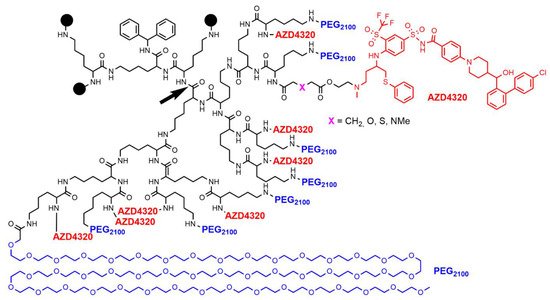
- Patterson, C.M.; Balachander, S.B.; Grant, I.; Pop-Damkov, P.; Kelly, B.; McCoull, W.; Parker, J.; Giannis, M.; Hill, K.J.; Gib-bons, F.D.; et al. Design and optimisation of dendrimer-conjugated Bcl-2/xL inhibitor, AZD0466, with improved therapeutic index for cancer therapy. Commun. Biol. 2021, 4, 112.
- Arulananda, S.; O’Brien, M.; Evangelista, M.; Jenkins, L.J.; Poh, A.R.; Walkiewicz, M.; Leong, T.; Mariadason, J.M.; Cebon, J.; Balachander, S.B.; et al. A novel BH3-mimetic, AZD0466, targeting BCL-XL and BCL-2 is effective in pre-clinical models of malignant pleural mesothelioma. Cell Death Discov. 2021, 7, 122.
- Feeney, O.M.; Ardipradja, K.; Noi, K.F.; Mehta, D.; De Rose, R.; Yuen, D.; Johnston, A.P.R.; Kingston, L.; Ericsson, C.; Elmore, C.S.; et al. Subcutaneous delivery of a dendrimer-BH3 mimetic improves lymphatic uptake and survival in lymphoma. J. Control. Release 2022, 348, 420–430.
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of poly-mers—Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132.
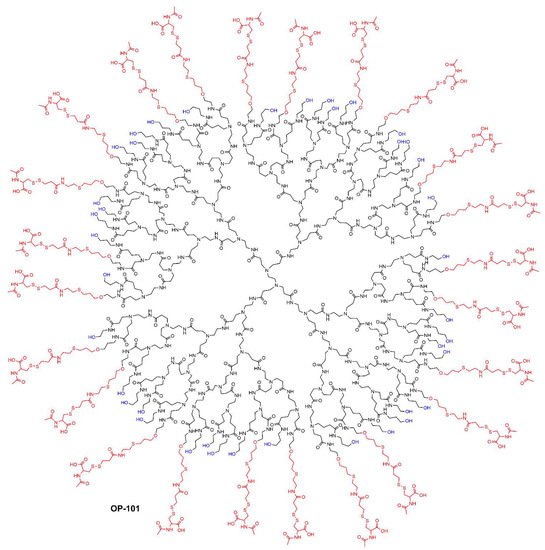
- Ashvattha Therapeutics. Available online: https://avttx.com/ (accessed on 2 August 2022).
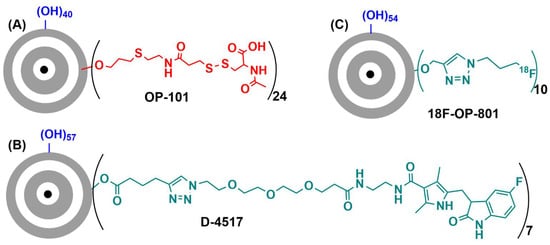
- Sharma, R.; Sharma, A.; Kambhampati, S.P.; Reddy, R.R.; Zhang, Z.; Cleland, J.L.; Kannan, S.; Kannan, R.M. Scalable syn-thesis and validation of PAMAM dendrimer-N-acetyl cysteine conjugate for potential translation. Bioeng. Transl. Med. 2018, 3, 87–101.
- Gusdon, A.M.; Faraday, N.; Aita, J.S.; Kumar, S.; Mehta, I.; Choi, H.A.; Cleland, J.L.; Robinson, K.; McCullough, L.D.; Ng, D.K.; et al. Dendrimer nanotherapy for severe COVID-19 attenuates inflammation and neurological injury markers and improves outcomes in a phase2a clinical trial. Sci. Transl. Med. 2022, 14, eabo2652.
- Kambhampati, S.P.; Bhutto, I.A.; Wu, T.; Ho, K.; McLeod, D.S.; Lutty, G.A.; Kannan, R.M. Systemic dendrimer nanotherapies for targeted suppression of choroidal inflammation and neovascularization in age-related macular degeneration. J. Control. Release 2021, 335, 527–540.
- Cleland, J.; Sharma, R.; Appiani, S. Dendrimer Compositions and Methods for Drug Delivery to the Eye. W O 2021/113662, 10 June 2021.
- Khaitov, M.R.; Shilovskii, I.P.; Kozhikhova, K.V.; Kofiadi, I.A.; Smirnov, V.V.; Koloskova, O.O.; Sergeev, I.V.; Trofimov, D.Y.; Trukhin, V.P.; Skvortsova, V.I. Combination Antiviral Formulation against SARS-CoV-2 Comprising SARS-CoV-2 Genome-Targeting siRNAs and Transfection-Enhancing Cationic Peptide Dendrimer. RU2746362 C1 2021-04-12, 11 March 2021.
- Khaitov, M.; Nikonova, A.; Shilovskiy, I.; Kozhikhova, K.; Kofiadi, I.; Vishnyakova, L.; Nikolskii, A.; Gattinger, P.; Kovchina, V.; Barvinskaia, E.; et al. Silencing of SARS-CoV-2 with modified siRNA-peptide dendrimer formulation. Allergy 2021, 76, 2840–2854.
