Administration of immunostimulants in fish is a preventive method to combat infections. A wide variety of these biological molecules exist, among which one of the yeast wall compounds stands out for its different biological activities. The β-glucan that forms the structural part of yeast is capable of generating immune activity in fish by cell receptor recognition. The most frequently used β-glucans for the study of mechanisms of action are those of commercial origin, with doses recommended by the manufacturer. Nevertheless, their immune activity is inefficient in some fish species, and increasing the dose may show adverse effects, including immunosuppression. Conversely, experimental β-glucans from other yeast species show different activities, such as antibacterial, antioxidant, healing, and stress tolerance properties.
- biomolecules
- functional carbohydrates
- immunity
- infectious diseases
1. Introduction
| Species | Mw * | Reference |
|---|---|---|
| Cystobasidium benthicum | 2.32 kDa | [22] |
| Saccharomyces cerevisiae (bakery) | 175 kDa | [23] |
| Saccharomyces uvarum | 220 kDa | [24] |
| Saccharomyces cerevisiae (brewery) | 240 kDa | [25] |
| Debaryomyces hansenii (BCS004) | 689.35 kDa | [26] |
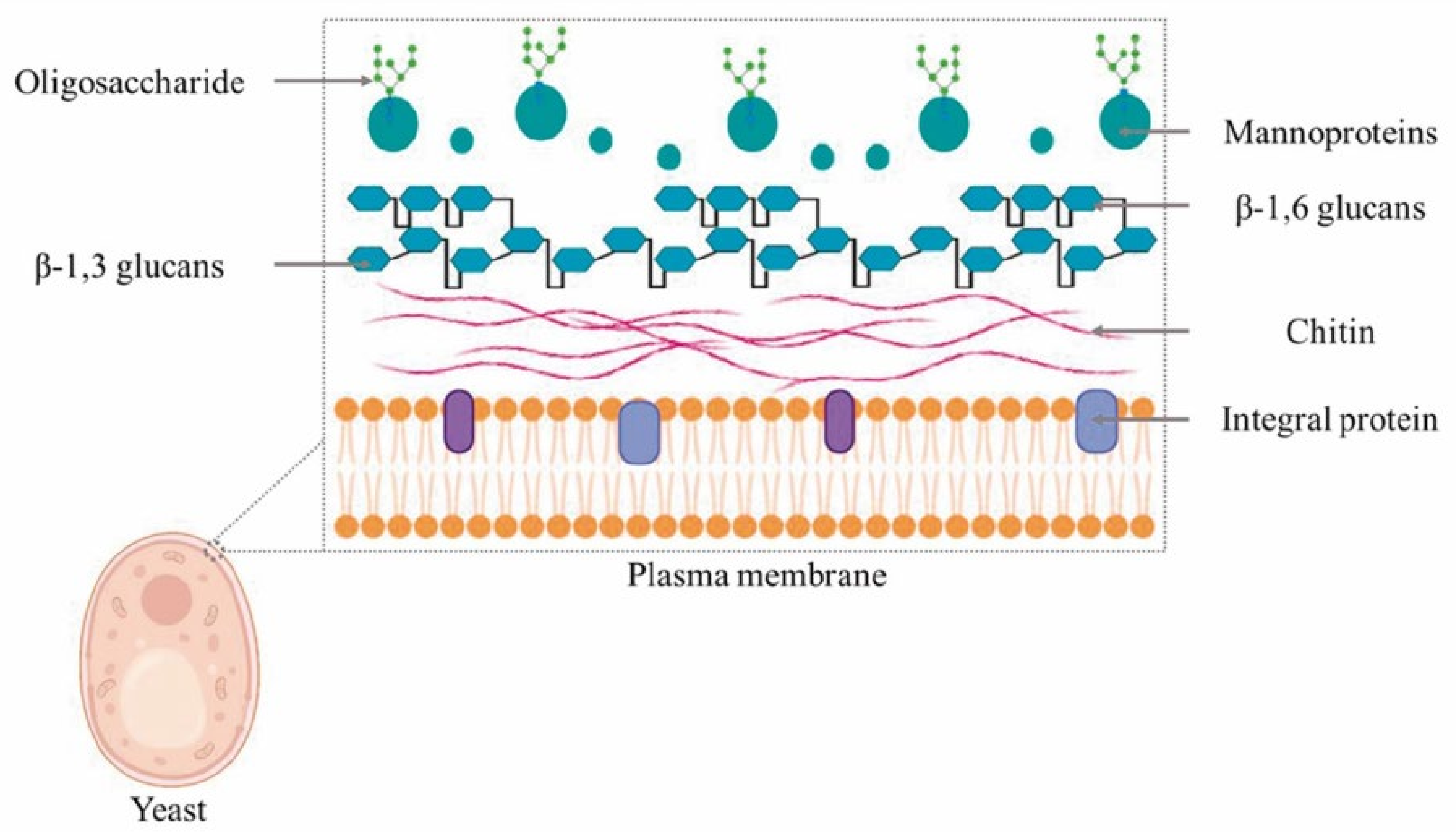
2. Yeast Cell Wall and β-Glucan Composition
2.1. Yeast Cell Wall
2.2. Yeast β-Glucans
3. Yeast β-Glucan Extraction
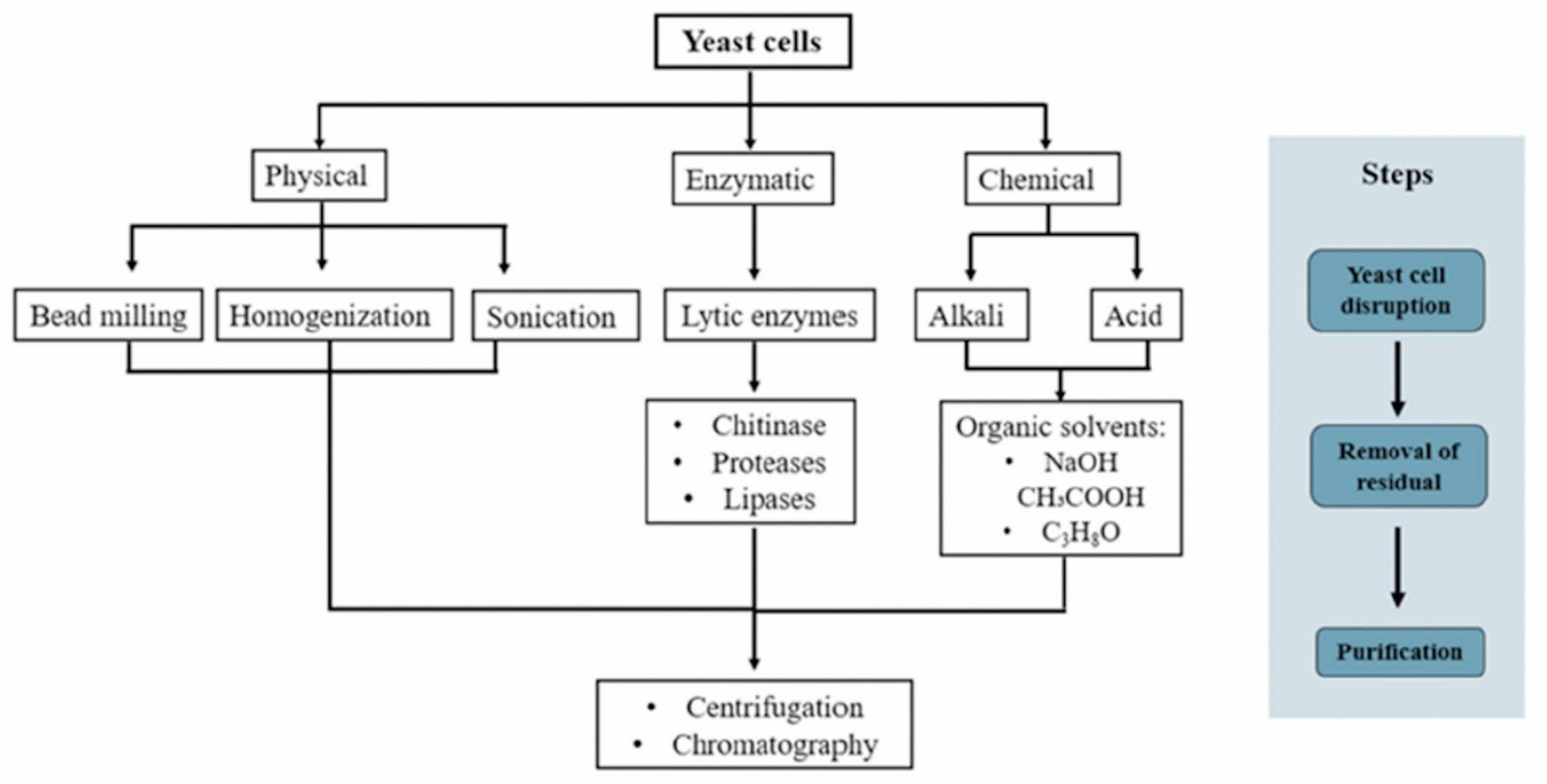
3.1. Physical Method
3.2. Chemical Method
3.3. Enzymatic Method
4. Effects of Yeast β-Glucans on Fish Immune System

This entry is adapted from the peer-reviewed paper 10.3390/ani12162154
References
- Øverland, M.; Skrede, A. Yeast derived from lignocellulosic biomass as a sustainable feed resource for use in aquaculture: Yeast from lignocellulosic biomass as a feed in aquaculture. J. Sci. Food Agric. 2016, 97, 733–742.
- Gianni, L. The fascinating and secret wild life of the budding yeast S. cerevisiae. eLife 2015, 4, e05835.
- Sagot, I.; Laporte, D. The cell biology of quiescent yeast—A diversity of individual scenarios. J. Cell Sci. 2019, 132, jcs213025.
- Martin, A.M.; Goddard, S.; Bemibster, P. Production of Candida utilis biomass as aquaculture feed. J. Sci. Food Agric. 1993, 61, 363–370.
- Reveco-Urzua, F.E.; Hofossæter, M.; Kovi, M.R.; Mydland, L.T.; Ånestad, R.; Sørby, R.; Press, C.M.; Lagos, L.; Øverland, M. Candida utilis yeast as a functional protein source for Atlantic salmon (Salmo salar L.): Local intestinal tissue and plasma proteome responses. PLoS ONE 2019, 14, e0218360.
- Dimitroglou, A.; Merrifield, D.L.; Spring, P.; Sweetman, J.; Moate, R.; Davies, S.J. Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata). Aquaculture 2010, 300, 182–188.
- Navarrete, P.; Tovar-Ramírez, D. Use of Yeasts as Probiotics in Fish Aquaculture. Sustain. Aquac. Tech. 2014, 1, 135–172.
- Gonçalves, A.; Gallardo-Escárate, C. Microbiome dynamic modulation through functional diets based on pre- and probiotics (mannan-oligosaccharides and Saccharomyces cerevisiae) in juvenile rainbow trout (Oncorhynchus mykiss). J. Appl. Microbiol. 2017, 122, 1333–1347.
- Yuan, X.-Y.; Liu, W.-B.; Liang, C.; Sun, C.-X.; Xue, Y.-F.; Wan, Z.-D.; Jiang, G.-Z. Effects of partial replacement of fish meal by yeast hydrolysate on complement system and stress resistance in juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 2017, 67, 312–321.
- Boonanuntanasarn, S.; Ditthab, K.; Jangprai, A.; Nakharuthai, C. Effects of Microencapsulated Saccharomyces cerevisiae on Growth, Hematological Indices, Blood Chemical, and Immune Parameters and Intestinal Morphology in Striped Catfish, Pangasianodon hypophthalmus. Probiotics Antimicrob. Proteins 2018, 11, 427–437.
- Voloski, A.P.D.S.; Soveral, L.D.F.; Dazzi, C.C.; Sutili, F.; Frandoloso, R.; Kreutz, L.C. β-Glucan improves wound healing in silver catfish (Rhamdia quelen). Fish Shellfish Immunol. 2019, 93, 575–579.
- Divya, M.; Gopi, N.; Iswarya, A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Vaseeharan, B. β-glucan extracted from eukaryotic single-celled microorganism Saccharomyces cerevisiae: Dietary supplementation and enhanced ammonia stress tolerance on Oreochromis mossambicus. Microb. Pathog. 2020, 139, 103917.
- Ji, L.; Fu, S.; Ji, R.; Li, X.; Liu, Y. β-glucan mitigated trinitrobenzene sulfonic acid-induced enteritis in the rainbow trout (Oncorhynchus mykiss). Aquaculture 2019, 513, 734393.
- Orlean, P. Architecture and Biosynthesis of the Saccharomyces cerevisiae Cell Wall. Genetics 2012, 192, 775–818.
- Aon, J.C.; Sun, J.; Leighton, J.M.; Appelbaum, E.R. Hypoxia-elicited impairment of cell wall integrity, glycosylation precursor synthesis, and growth in scaled-up high-cell density fed-batch cultures of Saccharomyces cerevisiae. Microb. Cell Factories 2016, 15, 1–16.
- Lesage, G.; Bussey, H. Cell Wall Assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343.
- Ozhovan, S.M.; Knorre, D.A.; Severin, F.F.; Bakeeva, L.E. Ultrastructure of yeast cell Saccharomyces cerevisiae after amiodarone treatment. Cell Tissue Biol. 2010, 4, 90–95.
- Reyes-Becerril, M.; Guardiola, F.A.; Sanchez, V.; Maldonado, M.; Angulo, C. Sterigmatomyces halophilus β-glucan improves the immune response and bacterial resistance in Pacific red snapper (Lutjanus peru) peripheral blood leucocytes: In vitro study. Fish Shellfish Immunol. 2018, 78, 392–403.
- Guzmán-Villanueva, L.T.; Ascencio-Valle, F.; Macías-Rodríguez, M.E.; Tovar-Ramírez, D. Effects of dietary β-1,3/1,6-glucan on the antioxidant and digestive enzyme activities of Pacific red snapper (Lutjanus peru) after exposure to lipopolysaccharides. Fish Physiol. Biochem. 2013, 40, 827–837.
- Elder, M.J.; Webster, S.J.; Chee, R.; Williams, D.L.; Gaston, J.S.H.; Goodall, J.C. β-Glucan Size Controls Dectin-1-Mediated Immune Responses in Human Dendritic Cells by Regulating IL-1β Production. Front. Immunol. 2017, 8, 791.
- Petit, J.; Wiegertjes, G.F. Long-lived effects of administering β-glucans: Indications for trained immunity in fish. Dev. Comp. Immunol. 2016, 64, 93–102.
- Reyes-Becerril, M.; Angulo, M.; Sanchez, V.; Machuca, C.; Méndez-Martínez, Y.; Angulo, C. β-Glucan bioactivities from Cystobasidium benthicum in Totoaba macdonaldi thymus cells. Fish Shellfish Immunol. 2021, 119, 542–553.
- Khan, A.A.; Gani, A.; Masoodi, F.; Amin, F.; Wani, I.A.; Khanday, F.A.; Gani, A. Structural, thermal, functional, antioxidant & antimicrobial properties of β- d -glucan extracted from baker’s yeast (Saccharomyces cereviseae)—Effect of γ-irradiation. Carbohydr. Polym. 2016, 140, 442–450.
- Araújo, V.B.D.S.; De Melo, A.N.F.; De Souza, N.T.; Da Silva, V.M.B.; Castro-Gomez, R.H.; Silva, A.S.; De Souza, E.L.; Magnani, M. Oral Intake of Carboxymethyl-Glucan (CM-G) from Yeast (Saccharomyces uvarum) Reduces Malondialdehyde Levels in Healthy Men. Molecules 2015, 20, 14950–14958.
- Yuan, H.; Lan, P.; He, Y.; Li, C.; Ma, X. Effect of the Modifications on the Physicochemical and Biological Properties of β-Glucan—A Critical Review. Molecules 2019, 25, 57.
- Reyes-Becerril, M.; Angulo, M.; Sanchez, V.; Guluarte, C.; Angulo, C. β-D-glucan from marine yeast Debaryomyces hansenii BCS004 enhanced intestinal health and glucan-expressed receptor genes in Pacific red snapper Lutjanus peru. Microb. Pathog. 2020, 143, 104141.
- Nguyen, T.H.; Fleet, G.H.; Rogers, P.L. Composition of the cell walls of several yeast species. Appl. Microbiol. Biotechnol. 1998, 50, 206–212.
- Leger-Silvestre, I.; Gas, N. The Nucleolus. In The Nucleolar Ultrastructure in Yeast; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2004; pp. 21–28.
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173.
- Bohn, J.A.; BeMiller, J.N. (1→3)-β-d-Glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydr. Polym. 1995, 28, 3–14.
- Gopalakannan, A.; Arul, V. Enhancement of the innate immune system and disease-resistant activity in Cyprinus carpio by oral administration of β-glucan and whole cell yeast. Aquac. Res. 2009, 41, 884–892.
- Adams, E.L.; Rice, P.J.; Graves, B.; Ensley, H.E.; Yu, H.; Brown, G.D.; Gordon, S.; Monteiro, M.A.; Papp-Szabo, E.; Lowman, D.W.; et al. Differential High-Affinity Interaction of Dectin-1 with Natural or Synthetic Glucans Is Dependent upon Primary Structure and Is Influenced by Polymer Chain Length and Side-Chain Branching. J. Pharmacol. Exp. Ther. 2008, 325, 115–123.
- Liu, Y.; Tang, Q.; Zhang, J.; Xia, Y.; Yang, Y.; Wu, D.; Fan, H.; Cui, S.W. Triple helix conformation of β-d-glucan from Ganoderma lucidum and effect of molecular weight on its immunostimulatory activity. Int. J. Biol. Macromol. 2018, 114, 1064–1070.
- Benito-Román, Ó.; Alonso, E.; Cocero, M.J. Ultrasound-assisted extraction of β-glucans from barley. LWT-Food Sci. Technol. 2013, 50, 57–63.
- Bystryak, S.; Santockyte, R.; Peshkovsky, A.S. Cell disruption of S. cerevisiae by scalable high-intensity ultrasound. Biochem. Eng. J. 2015, 99, 99–106.
- Sourki, A.H.; Koocheki, A.; Elahi, M. Ultrasound-assisted extraction of β-d-glucan from hull-less barley: Assessment of physicochemical and functional properties. Int. J. Biol. Macromol. 2017, 95, 462–475.
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Ahmed, Z. Extraction and characterization of β-d-glucan from oat for industrial utilization. Int. J. Biol. Macromol. 2010, 46, 304–309.
- Islam, M.S.; Aryasomayajula, A.; Selvaganapathy, P.R. A Review on Macroscale and Microscale Cell Lysis Methods. Micromachines 2017, 8, 83.
- Varelas, V.; Liouni, M.; Calokerinos, A.C.; Nerantzis, E.T. An evaluation study of different methods for the production of β-D-glucan from yeast biomass. Drug Test. Anal. 2015, 8, 46–55.
- Javmen, A.; Grigiskis, S.; Gliebutė, R. β-glucan extraction from Saccharomyces cerevisiae yeast using Actinomyces rutgersensis 88 yeast lyzing enzymatic complex. Biologija 2012, 58, 51–59.
- Dallies, N.; François, J.; Paquet, V. A new method for quantitative determination of polysaccharides in the yeast cell wall. Application to the cell wall defective mutants of Saccharomyces cerevisiae. Yeast 1998, 14, 1297–1306.
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288.
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41.
- Pionnier, N.; Falco, A.; Miest, J.J.; Shrive, A.K.; Hoole, D. Feeding common carp Cyprinus carpio with β-glucan supplemented diet stimulates C-reactive protein and complement immune acute phase responses following PAMPs injection. Fish Shellfish Immunol. 2014, 39, 285–295.
- Kiron, V.; Kulkarni, A.; Dahle, D.; Vasanth, G.; Lokesh, J.; Elvebo, O. Recognition of purified beta 1,3/1,6 glucan and molecular signalling in the intestine of Atlantic salmon. Dev. Comp. Immunol. 2016, 56, 57–66.
- Lauriano, E.; Pergolizzi, S.; Capillo, G.; Kuciel, M.; Alesci, A.; Faggio, C. Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol. 2016, 59, 250–255.
- Pietretti, D.; Vera-Jimenez, N.; Hoole, D.; Wiegertjes, G. Oxidative burst and nitric oxide responses in carp macrophages induced by zymosan, MacroGard® and selective dectin-1 agonists suggest recognition by multiple pattern recognition receptors. Fish Shellfish Immunol. 2013, 35, 847–857.
- Angulo, C.; Sanchez, V.; Delgado, K.; Reyes-Becerril, M. C-type lectin 17A and macrophage-expressed receptor genes are magnified by fungal β-glucan after Vibrio parahaemolyticus infection in Totoaba macdonaldi cells. Immunobiology 2018, 224, 102–109.
- Ji, L.; Sun, G.; Li, X.; Liu, Y. Comparative transcriptome analysis reveals the mechanism of β-glucan in protecting rainbow trout (Oncorhynchus mykiss) from Aeromonas salmonicida infection. Fish Shellfish Immunol. 2019, 98, 87–99.
- Mikrou, A.; Marioli, D.; Papanastasiou, A.D.; Zarkadis, I.K. CR3 complement receptor: Cloning and characterization in rainbow trout. Fish Shellfish Immunol. 2009, 26, 19–28.
- Zhang, C.; Li, C.; Jia, X.; Wang, K.; Tu, Y.; Wang, R.; Liu, K.; Lu, T.; He, C. In Vitro and In Vivo Anti-Inflammatory Effects of Polyphyllin VII through Downregulating MAPK and NF-κB Pathways. Molecules 2019, 24, 875.
- Wei, X.; Li, B.; Wu, L.; Yin, X.; Zhong, X.; Li, Y.; Wang, Y.; Guo, Z.; Ye, J. Interleukin-6 gets involved in response to bacterial infection and promotes antibody production in Nile tilapia (Oreochromis niloticus). Dev. Comp. Immunol. 2018, 89, 141–151.
- Huo, H.J.; Chen, S.N.; Li, L.; Nie, P. Functional characterization of IL-10 and its receptor subunits in a perciform fish, the mandarin fish, Siniperca chuatsi. Dev. Comp. Immunol. 2019, 97, 64–75.
- Zhu, Q.; Fan, Z.-J.; Cai, S.-X.; Yao, C.-L. Molecular and immunological characterizations of interleukin-11 in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2020, 100, 9–17.
- Piazzon, M.C.; Galindo-Villegas, J.; Pereiro, P.; Estensoro, I.; Calduch-Giner, J.A.; Gómez-Casado, E.; Novoa, B.; Mulero, V.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Differential Modulation of IgT and IgM upon Parasitic, Bacterial, Viral, and Dietary Challenges in a Perciform Fish. Front. Immunol. 2016, 7, 637.
- Danilova, N.; Bussmann, J.; Jekosch, K.; Steiner, L.A. The immunoglobulin heavy-chain locus in zebrafish: Identification and expression of a previously unknown isotype, immunoglobulin Z. Nat. Immunol. 2005, 6, 295–302.
- Hansen, J.D.; Landis, E.D.; Phillips, R.B. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proc. Natl. Acad. Sci. USA 2005, 102, 6919–6924.
- Zhang, Y.-A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; La Patra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835.
- Salah, A.S.; El Nahas, A.F.; Mahmoud, S. Modulatory effect of different doses of β-1,3/1,6-glucan on the expression of antioxidant, inflammatory, stress and immune-related genes of Oreochromis niloticus challenged with Streptococcus iniae. Fish Shellfish Immunol. 2017, 70, 204–213.
- Angulo, C.; Alamillo, E.; Ascencio, F.; Reyes-Becerril, M. Characterization of nuclear factor of activated T-cells-c3 (NFATc3) and gene expression of upstream-downstream signaling molecules in response to immunostimulants in Pacific red snapper cells. Dev. Comp. Immunol. 2018, 78, 149–159.
- Alamillo, E.; Reyes-Becerril, M.; Cuesta, A.; Angulo, C. Marine yeast Yarrowia lipolytica improves the immune responses in Pacific red snapper (Lutjanus peru) leukocytes. Fish Shellfish Immunol. 2017, 70, 48–56.
