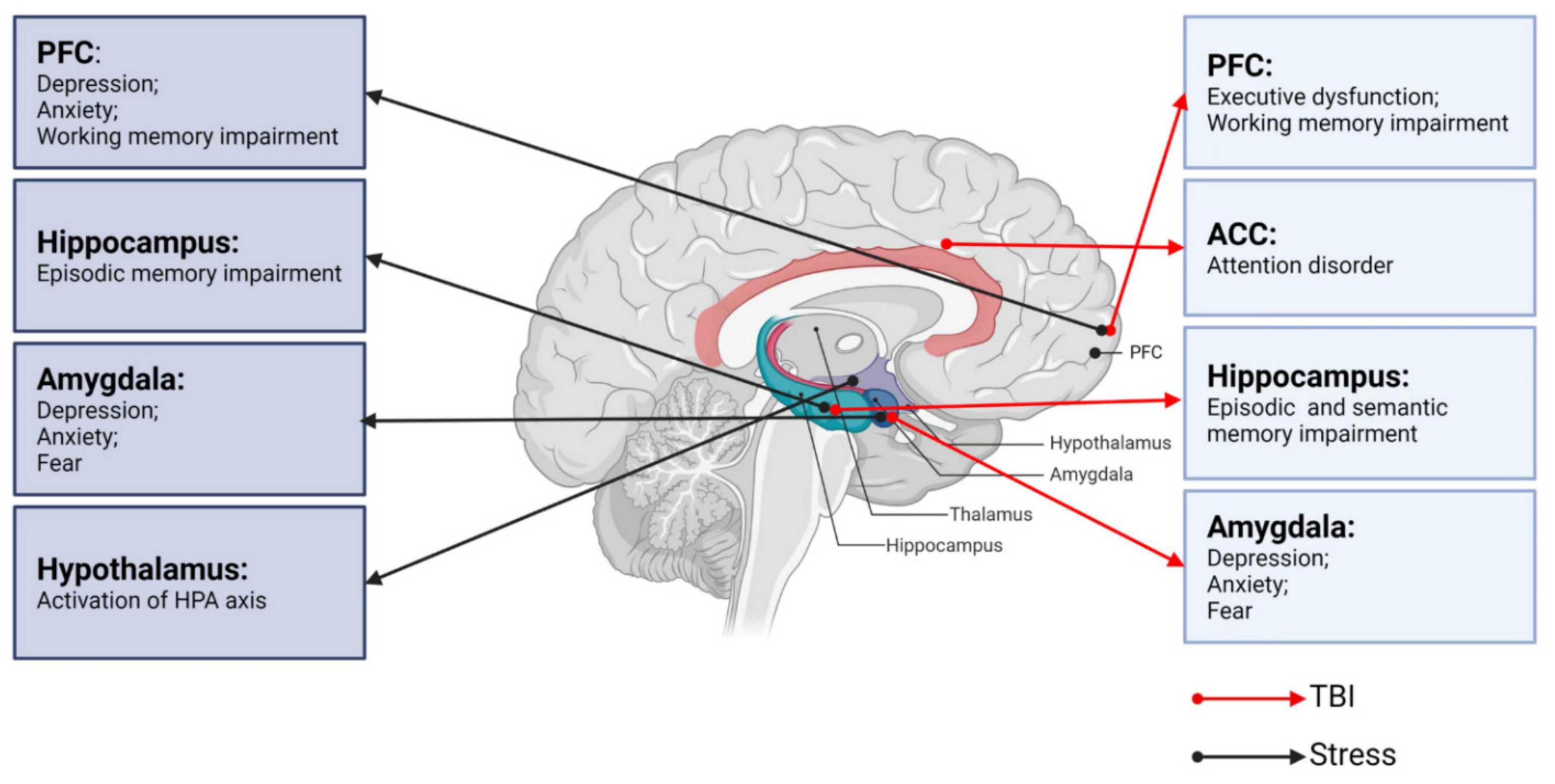
Neurological dysfunctions commonly occurs after mild or moderate traumatic brain injury (TBI). Although most TBI patients recover from such dysfunction in a short period of time, some present with persistent neurological deficits. Stress is a potential factor that is involved in recovery from neurological dysfunction after TBI. However, there has been limited research on the effects and mechanisms of stress on neurological dysfunctions due to TBI. The effects of TBI and stress on neurological dysfunctions and different brain regions such as the prefrontal cortex, hippocampus, amygdala, and hypothalamus are investigated, and the neurobiological links and mechanisms between stress and TBI are explored.
- traumatic brain injury
- stress
- brain region
- neurological dysfunction
- biomarker
1. Introduction
2. Neurobiological Links between TBI and Neurological Dysfunctions
2.1. TBI and Cognitive Impairments
2.2. TBI and Emotional/Behavioral Disturbances
3. Neurobiological Links between Stress and Key Brain Regions
3.1. Stress and the HPA Axis
3.2. Stress and the LC-NE System
3.3. Stress and the PFC
3.4. Stress and Hippocampus
3.5. Stress and Amygdala
This entry is adapted from the peer-reviewed paper 10.3390/ijms23179519
References
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048.
- Feigin, V.L.; Theadom, A.; Barker-Collo, S.; Starkey, N.J.; McPherson, K.; Kahan, M.; Dowell, A.; Brown, P.; Parag, V.; Kydd, R.; et al. Incidence of traumatic brain injury in New Zealand: A population-based study. Lancet Neurol. 2013, 12, 53–64.
- Jiang, J.-Y.; Gao, G.-Y.; Feng, J.-F.; Mao, Q.; Chen, L.-G.; Yang, X.-F.; Liu, J.-F.; Wang, Y.-H.; Qiu, B.-H.; Huang, X.-J. Traumatic brain injury in China. Lancet Neurol. 2019, 18, 286–295.
- Howe, E.I.; Langlo, K.S.; Terjesen, H.C.A.; Røe, C.; Schanke, A.K.; Søberg, H.L.; Sveen, U.; Aas, E.; Enehaug, H.; Alves, D.E.; et al. Combined cognitive and vocational interventions after mild to moderate traumatic brain injury: Study protocol for a randomized controlled trial. Trials 2017, 18, 483.
- Markovic, S.J.; Fitzgerald, M.; Peiffer, J.J.; Scott, B.R.; Rainey-Smith, S.R.; Sohrabi, H.R.; Brown, B.M. The impact of exercise, sleep, and diet on neurocognitive recovery from mild traumatic brain injury in older adults: A narrative review. Ageing Res. Rev. 2021, 68, 101322.
- Fischer, J.T.; Bickart, K.C.; Giza, C.; Babikian, T. A Review of Family Environment and Neurobehavioral Outcomes Following Pediatric Traumatic Brain Injury: Implications of Early Adverse Experiences, Family Stress, and Limbic Development. Biol. Psychiatry 2022, 91, 488–497.
- Zhang, J.Y.; Liu, T.H.; He, Y.; Pan, H.Q.; Zhang, W.H.; Yin, X.P.; Tian, X.L.; Li, B.M.; Wang, X.D.; Holmes, A.; et al. Chronic Stress Remodels Synapses in an Amygdala Circuit-Specific Manner. Biol. Psychiatry 2019, 85, 189–201.
- Merino, E.; Raya-Salom, D.; Teruel-Martí, V.; Adell, A.; Cervera-Ferri, A.; Martínez-Ricós, J. Effects of Acute Stress on the Oscillatory Activity of the Hippocampus–Amygdala–Prefrontal Cortex Network. Neuroscience 2021, 476, 72–89.
- Kazakou, P.; Nicolaides, N.C.; Chrousos, G.P. Basic Concepts and Hormonal Regulators of the Stress System. Horm. Res. Paediatr. 2022.
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640.
- Pavlovic, D.; Pekic, S.; Stojanovic, M.; Popovic, V. Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 2019, 22, 270–282.
- Andriessen, T.M.; Jacobs, B.; Vos, P.E. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J. Cell. Mol. Med. 2010, 14, 2381–2392.
- Pudenz, R.H.; Shelden, C.H. The Lucite Calvarium—A Method for Direct Observation of the Brain: II. Cranial Trauma and Brain Movement. J. Neurosurg. 1946, 3, 487–505.
- McGinn, M.J.; Povlishock, J.T. Pathophysiology of Traumatic Brain Injury. Neurosurg. Clin. N. Am. 2016, 27, 397–407.
- Stocchetti, N.; Carbonara, M.; Citerio, G.; Ercole, A.; Skrifvars, M.B.; Smielewski, P.; Zoerle, T.; Menon, D.K. Severe traumatic brain injury: Targeted management in the intensive care unit. Lancet Neurol. 2017, 16, 452–464.
- Pearn, M.L.; Niesman, I.R.; Egawa, J.; Sawada, A.; Almenar-Queralt, A.; Shah, S.B.; Duckworth, J.L.; Head, B.P. Pathophysiology Associated with Traumatic Brain Injury: Current Treatments and Potential Novel Therapeutics. Cell. Mol. Neurobiol. 2017, 37, 571–585.
- Wang, K.; Cui, D.M.; Gao, L. Traumatic brain injury: A review of characteristics, molecular basis and management. Front. Biosci. 2016, 21, 890–899.
- Sullivan, P.G.; Rabchevsky, A.G.; Waldmeier, P.C.; Springer, J.E. Mitochondrial permeability transition in CNS trauma: Cause or effect of neuronal cell death? J. Neurosci. Res. 2005, 79, 231–239.
- Khatri, N.; Thakur, M.; Pareek, V.; Kumar, S.; Sharma, S.; Datusalia, K.A. Oxidative Stress: Major Threat in Traumatic Brain Injury. CNS Neurol. Disord. Drug Targets 2018, 17, 689–695.
- Anthonymuthu, T.S.; Kenny, E.M.; Bayır, H. Therapies targeting lipid peroxidation in traumatic brain injury. Brain Res. 2016, 1640, 57–76.
- Ladak, A.A.; Enam, S.A.; Ibrahim, M.T. A Review of the Molecular Mechanisms of Traumatic Brain Injury. World Neurosurg. 2019, 131, 126–132.
- Akamatsu, Y.; Hanafy, K.A. Cell Death and Recovery in Traumatic Brain Injury. Neurotherapeutics 2020, 17, 446–456.
- Wehn, A.C.; Khalin, I.; Duering, M.; Hellal, F.; Culmsee, C.; Vandenabeele, P.; Plesnila, N.; Terpolilli, N.A. RIPK1 or RIPK3 deletion prevents progressive neuronal cell death and improves memory function after traumatic brain injury. Acta Neuropathol. Commun. 2021, 9, 138.
- Walker, K.R.; Tesco, G. Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front. Aging Neurosci. 2013, 5, 29.
- Teasdale, G.; Jennett, B. Assessment of Coma and Impaired Consciousness. Lancet 1974, 304, 81–84.
- Friedland, D.; Swash, M. Post-traumatic amnesia and confusional state: Hazards of retrospective assessment. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1068–1074.
- Rao, V.; Syeda, A.; Roy, D.; Peters, M.E.; Vaishnavi, S. Neuropsychiatric aspects of concussion: Acute and chronic sequelae. Concussion 2017, 2, CNC29.
- Tucker, L.B.; Velosky, A.G.; McCabe, J.T. Applications of the Morris water maze in translational traumatic brain injury research. Neurosci. Biobehav. Rev. 2018, 88, 187–200.
- Stocchetti, N.; Zanier, E.R. Chronic impact of traumatic brain injury on outcome and quality of life: A narrative review. Crit. Care 2016, 20, 148.
- Rabinowitz, A.R.; Levin, H.S. Cognitive sequelae of traumatic brain injury. Psychiatr. Clin. N. Am. 2014, 37, 1–11.
- Funahashi, S. Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci. Res. 2001, 39, 147–165.
- Levin, H.S.; Hanten, G. Executive functions after traumatic brain injury in children. Pediatr. Neurol. 2005, 33, 79–93.
- Wood, R.L.; Worthington, A. Neurobehavioral Abnormalities Associated with Executive Dysfunction after Traumatic Brain Injury. Front. Behav. Neurosci. 2017, 11, 195.
- Alexander, G.E.; DeLong, M.R.; Strick, P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986, 9, 357–381.
- De Simoni, S.; Jenkins, P.O.; Bourke, N.J.; Fleminger, J.J.; Hellyer, P.J.; Jolly, A.E.; Patel, M.C.; Cole, J.H.; Leech, R.; Sharp, D.J. Altered caudate connectivity is associated with executive dysfunction after traumatic brain injury. Brain 2018, 141, 148–164.
- Bamford, I.J.; Bamford, N.S. The Striatum’s Role in Executing Rational and Irrational Economic Behaviors. Neuroscientist 2019, 25, 475–490.
- Ketchesin, K.D.; Zong, W.; Hildebrand, M.A.; Seney, M.L.; Cahill, K.M.; Scott, M.R.; Shankar, V.G.; Glausier, J.R.; Lewis, D.A.; Tseng, G.C.; et al. Diurnal rhythms across the human dorsal and ventral striatum. Proc. Natl. Acad. Sci. USA 2021, 118, e2016150118.
- McColgan, P.; Seunarine, K.K.; Razi, A.; Cole, J.H.; Gregory, S.; Durr, A.; Roos, R.A.; Stout, J.C.; Landwehrmeyer, B.; Scahill, R.I.; et al. Selective vulnerability of Rich Club brain regions is an organizational principle of structural connectivity loss in Huntington’s disease. Brain 2015, 138, 3327–3344.
- Ndayisaba, A.; Jellinger, K.; Berger, T.; Wenning, G.K. TNFα inhibitors as targets for protective therapies in MSA: A viewpoint. J. Neuroinflamm. 2019, 16, 80.
- Elliott, R. Executive functions and their disorders: Imaging in clinical neuroscience. Br. Med. Bull. 2003, 65, 49–59.
- Mennes, M.; Vega Potler, N.; Kelly, C.; Di Martino, A.; Castellanos, F.X.; Milham, M.P. Resting state functional connectivity correlates of inhibitory control in children with attention-deficit/hyperactivity disorder. Front. Psychiatry 2011, 2, 83.
- Paterno, R.; Folweiler, K.A.; Cohen, A.S. Pathophysiology and Treatment of Memory Dysfunction after Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2017, 17, 52.
- Vakil, E.; Greenstein, Y.; Weiss, I.; Shtein, S. The Effects of Moderate-to-Severe Traumatic Brain Injury on Episodic Memory: A Meta-Analysis. Neuropsychol. Rev. 2019, 29, 270–287.
- Le Fur, C.; Câmara-Costa, H.; Francillette, L.; Opatowski, M.; Toure, H.; Brugel, D.; Laurent-Vannier, A.; Meyer, P.; Watier, L.; Dellatolas, G.; et al. Executive functions and attention 7years after severe childhood traumatic brain injury: Results of the Traumatisme Grave de l’Enfant (TGE) cohort. Ann. Phys. Rehabil. Med. 2020, 63, 270–279.
- Ornstein, T.J.; Sagar, S.; Schachar, R.J.; Ewing-Cobbs, L.; Chapman, S.B.; Dennis, M.; Saunders, A.E.; Yang, T.T.; Levin, H.S.; Max, J.E. Neuropsychological performance of youth with secondary attention-deficit/hyperactivity disorder 6- and 12-months after traumatic brain injury. J. Int. Neuropsychol. Soc. 2014, 20, 971–981.
- Anderson, V.; Eren, S.; Dob, R.; Le Brocque, R.; Iselin, G.; Davern, T.J.; McKinlay, L.; Kenardy, J. Early Attention Impairment and Recovery Profiles After Childhood Traumatic Brain Injury. J. Head Trauma Rehabil. 2012, 27, 199–209.
- Shah, S.A.; Goldin, Y.; Conte, M.M.; Goldfine, A.M.; Mohamadpour, M.; Fidali, B.C.; Cicerone, K.; Schiff, N.D. Executive attention deficits after traumatic brain injury reflect impaired recruitment of resources. NeuroImage Clin. 2017, 14, 233–241.
- Cazalis, F.; Babikian, T.; Giza, C.; Copeland, S.; Hovda, D.; Asarnow, R.F. Pivotal role of anterior cingulate cortex in working memory after traumatic brain injury in youth. Front. Neurol. 2011, 1, 158.
- Xuan, B.; Mackie, M.A.; Spagna, A.; Wu, T.; Tian, Y.; Hof, P.R.; Fan, J. The activation of interactive attentional networks. Neuroimage 2016, 129, 308–319.
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222.
- Schneider, K.N.; Sciarillo, X.A.; Nudelman, J.L.; Cheer, J.F.; Roesch, M.R. Anterior Cingulate Cortex Signals Attention in a Social Paradigm that Manipulates Reward and Shock. Curr. Biol. 2020, 30, 3724–3735.e2.
- Sheth, C.; Rogowska, J.; Legarreta, M.; McGlade, E.; Yurgelun-Todd, D. Functional connectivity of the anterior cingulate cortex in Veterans with mild traumatic brain injury. Behav. Brain Res. 2021, 396, 112882.
- Whiting, M.D.; Hamm, R.J. Mechanisms of anterograde and retrograde memory impairment following experimental traumatic brain injury. Brain Res. 2008, 1213, 69–77.
- Cowan, N. Chapter 20 What are the differences between long-term, short-term, and working memory? In Essence of Memory; Elsevier: Amsterdam, The Netherlands, 2008; pp. 323–338.
- Fortier-Lebel, O.; Jobin, B.; Lecuyer-Giguere, F.; Gaubert, M.; Giguere, J.F.; Gagnon, J.F.; Boller, B.; Frasnelli, J. Verbal Episodic Memory Alterations and Hippocampal Atrophy in Acute Mild Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1506–1514.
- Klooster, N.B.; Tranel, D.; Duff, M.C. The hippocampus and semantic memory over time. Brain Lang. 2020, 201, 104711.
- Manns, J.R.; Hopkins, R.O.; Squire, L.R. Semantic Memory and the Human Hippocampus. Neuron 2003, 38, 127–133.
- Van der Horn, H.J.; Liemburg, E.J.; Scheenen, M.E.; de Koning, M.E.; Marsman, J.B.; Spikman, J.M.; van der Naalt, J. Brain network dysregulation, emotion, and complaints after mild traumatic brain injury. Hum. Brain Mapp. 2016, 37, 1645–1654.
- Simic, G.; Tkalcic, M.; Vukic, V.; Mulc, D.; Spanic, E.; Sagud, M.; Olucha-Bordonau, F.E.; Vuksic, M.; Hof, P.R. Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules 2021, 11, 823.
- Han, K.; Chapman, S.B.; Krawczyk, D.C. Altered Amygdala Connectivity in Individuals with Chronic Traumatic Brain Injury and Comorbid Depressive Symptoms. Front. Neurol. 2015, 6, 231.
- Young, L.R.; Yu, W.; Holloway, M.; Rodgers, B.N.; Chapman, S.B.; Krawczyk, D.C. Amygdala activation as a marker for selective attention toward neutral faces in a chronic traumatic brain injury population. Neuropsychologia 2017, 104, 214–222.
- Celeghin, A.; Galetto, V.; Tamietto, M.; Zettin, M. Emotion Recognition in Low-Spatial Frequencies Is Partly Preserved following Traumatic Brain Injury. BioMed Res. Int. 2019, 2019, 9562935.
- Lim, S.W.; Shiue, Y.L.; Liao, J.C.; Wee, H.Y.; Wang, C.C.; Chio, C.C.; Chang, C.H.; Hu, C.Y.; Kuo, J.R. Simvastatin Therapy in the Acute Stage of Traumatic Brain Injury Attenuates Brain Trauma-Induced Depression-Like Behavior in Rats by Reducing Neuroinflammation in the Hippocampus. Neurocrit. Care 2017, 26, 122–132.
- Silver, J.M.; McAllister, T.W.; Arciniegas, D.B. Depression and Cognitive Complaints Following Mild Traumatic Brain Injury. Am. J. Psychiatry 2009, 166, 653–661.
- Anand, A.; Li, Y.; Wang, Y.; Wu, J.; Gao, S.; Bukhari, L.; Mathews, V.P.; Kalnin, A.; Lowe, M.J. Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biol. Psychiatry 2005, 57, 1079–1088.
- Matthews, S.C.; Strigo, I.A.; Simmons, A.N.; Yang, T.T.; Paulus, M.P. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J. Affect. Disord. 2008, 111, 13–20.
- Chen, C.H.; Suckling, J.; Ooi, C.; Fu, C.H.; Williams, S.C.; Walsh, N.D.; Mitterschiffthaler, M.T.; Pich, E.M.; Bullmore, E. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology 2008, 33, 1909–1918.
- Beitchman, J.A.; Griffiths, D.R.; Hur, Y.; Ogle, S.B.; Bromberg, C.E.; Morrison, H.W.; Lifshitz, J.; Adelson, P.D.; Thomas, T.C. Experimental Traumatic Brain Injury Induces Chronic Glutamatergic Dysfunction in Amygdala Circuitry Known to Regulate Anxiety-Like Behavior. Front. Neurosci. 2019, 13, 1434.
- Figueiredo, T.H.; Harbert, C.L.; Pidoplichko, V.; Almeida-Suhett, C.P.; Pan, H.; Rossetti, K.; Braga, M.F.M.; Marini, A.M. Alpha-Linolenic Acid Treatment Reduces the Contusion and Prevents the Development of Anxiety-Like Behavior Induced by a Mild Traumatic Brain Injury in Rats. Mol. Neurobiol. 2018, 55, 187–200.
- Treadway, M.T.; Waskom, M.L.; Dillon, D.G.; Holmes, A.J.; Park, M.T.M.; Chakravarty, M.M.; Dutra, S.J.; Polli, F.E.; Iosifescu, D.V.; Fava, M.; et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol. Psychiatry 2015, 77, 285–294.
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017, 6871089.
- Jang, S.H.; Yi, J.H.; Kwon, H.G. Injury of the dorsolateral prefronto-thalamic tract in a patient with depression following mild traumatic brain injury: A case report. Medicine 2016, 95, e5009.
- Palacios, E.M.; Sala-Llonch, R.; Junque, C.; Fernandez-Espejo, D.; Roig, T.; Tormos, J.M.; Bargallo, N.; Vendrell, P. Long-term declarative memory deficits in diffuse TBI: Correlations with cortical thickness, white matter integrity and hippocampal volume. Cortex 2013, 49, 646–657.
- Bae, S.; Sheth, C.; Legarreta, M.; McGlade, E.; Lyoo, I.K.; Yurgelun-Todd, D.A. Volume and shape analysis of the Hippocampus and amygdala in veterans with traumatic brain injury and posttraumatic stress disorder. Brain Imaging Behav. 2020, 14, 1850–1864.
- Janak, P.H.; Tye, K.M. From circuits to behaviour in the amygdala. Nature 2015, 517, 284–292.
- Babaev, O.; Piletti Chatain, C.; Krueger-Burg, D. Inhibition in the amygdala anxiety circuitry. Exp. Mol. Med. 2018, 50, 1–16.
- Orsini, C.A.; Maren, S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci. Biobehav. Rev. 2012, 36, 1773–1802.
- Tye, K.M.; Prakash, R.; Kim, S.Y.; Fenno, L.E.; Grosenick, L.; Zarabi, H.; Thompson, K.R.; Gradinaru, V.; Ramakrishnan, C.; Deisseroth, K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 2011, 471, 358–362.
- Agoglia, A.E.; Herman, M.A. The center of the emotional universe: Alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018, 72, 61–73.
- LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000, 23, 155–184.
- Duvarci, S.; Pare, D. Amygdala microcircuits controlling learned fear. Neuron 2014, 82, 966–980.
- Ehrlich, I.; Humeau, Y.; Grenier, F.; Ciocchi, S.; Herry, C.; Luthi, A. Amygdala inhibitory circuits and the control of fear memory. Neuron 2009, 62, 757–771.
- Makkar, S.R.; Zhang, S.Q.; Cranney, J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology 2010, 35, 1625–1652.
- Shi, Y.; Wu, X.; Zhou, J.; Cui, W.; Wang, J.; Hu, Q.; Zhang, S.; Han, L.; Zhou, M.; Luo, J.; et al. Single-Nucleus RNA Sequencing Reveals that Decorin Expression in the Amygdala Regulates Perineuronal Nets Expression and Fear Conditioning Response after Traumatic Brain Injury. Adv. Sci. 2022, 9, e2104112.
- Ramlackhansingh, A.F.; Brooks, D.J.; Greenwood, R.J.; Bose, S.K.; Turkheimer, F.E.; Kinnunen, K.M.; Gentleman, S.; Heckemann, R.A.; Gunanayagam, K.; Gelosa, G.; et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann. Neurol. 2011, 70, 374–383.
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013, 136, 28–42.
- The National Academies of Sciences, Engineering, and Medicine. Evaluation of the Disability Determination Process for Traumatic Brain Injury in Veterans; The National Academies Press: Washington, DC, USA, 2019; p. 210.
- Bay, E.; de-Leon, M.B. Chronic Stress and Fatigue-Related Quality of Life After Mild to Moderate Traumatic Brain Injury. J. Head Trauma Rehabil. 2011, 26, 355–363.
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381.
- Dragoş, D.; Tănăsescu, M.D. The effect of stress on the defense systems. J. Med. Life 2010, 3, 10–18.
- McEwen, B.S.; Gianaros, P.J. Stress- and allostasis-induced brain plasticity. Annu. Rev. Med. 2011, 62, 431–445.
- Joëls, M.; Pu, Z.; Wiegert, O.; Oitzl, M.S.; Krugers, H.J. Learning under stress: How does it work? Trends Cogn. Sci. 2006, 10, 152–158.
- Liston, C.; Miller, M.M.; Goldwater, D.S.; Radley, J.J.; Rocher, A.B.; Hof, P.R.; Morrison, J.H.; McEwen, B.S. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006, 26, 7870–7874.
- Gameiro, G.H.; Gameiro, P.H.; da Silva Andrade, A.; Pereira, L.F.; Arthuri, M.T.; Marcondes, F.K.; de Arruda Veiga, M.C.F. Nociception- and anxiety-like behavior in rats submitted to different periods of restraint stress. Physiol. Behav. 2006, 87, 643–649.
- Buynitsky, T.; Mostofsky, D.I. Restraint stress in biobehavioral research: Recent developments. Neurosci. Biobehav. Rev. 2009, 33, 1089–1098.
- McEwen, B.S.; Gianaros, P.J. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010, 1186, 190–222.
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621.
- Herman, J.P.; Tasker, J.G. Paraventricular Hypothalamic Mechanisms of Chronic Stress Adaptation. Front. Endocrinol. 2016, 7, 137.
- Joels, M. Corticosteroids and the brain. J. Endocrinol. 2018, 238, R121–R130.
- Jankord, R.; Herman, J.P. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann. N. Y. Acad. Sci. 2008, 1148, 64–73.
- Ding, X.F.; Zhao, X.H.; Tao, Y.; Zhong, W.C.; Fan, Q.; Diao, J.X.; Liu, Y.L.; Chen, Y.Y.; Chen, J.X.; Lv, Z.P. Xiao Yao San Improves Depressive-Like Behaviors in Rats with Chronic Immobilization Stress through Modulation of Locus Coeruleus-Norepinephrine System. Evid. Based Complement. Altern. Med. 2014, 2014, 605914.
- Schwarz, L.A.; Luo, L. Organization of the locus coeruleus-norepinephrine system. Curr. Biol. 2015, 25, R1051–R1056.
- Arnsten, A.F. Through the looking glass: Differential noradenergic modulation of prefrontal cortical function. Neural Plast. 2000, 7, 133–146.
- Benarroch, E.E. Locus coeruleus. Cell Tissue Res. 2018, 373, 221–232.
- Harris, K.D.; Shepherd, G.M.G. The neocortical circuit: Themes and variations. Nat. Neurosci. 2015, 18, 170–181.
- McKlveen, J.M.; Moloney, R.D.; Scheimann, J.R.; Myers, B.; Herman, J.P. “Braking” the Prefrontal Cortex: The Role of Glucocorticoids and Interneurons in Stress Adaptation and Pathology. Biol. Psychiatry 2019, 86, 669–681.
- McKlveen, J.M.; Myers, B.; Herman, J.P. The medial prefrontal cortex: Coordinator of autonomic, neuroendocrine and behavioural responses to stress. J. Neuroendocrinol. 2015, 27, 446–456.
- McKlveen, J.M.; Morano, R.L.; Fitzgerald, M.; Zoubovsky, S.; Cassella, S.N.; Scheimann, J.R.; Ghosal, S.; Mahbod, P.; Packard, B.A.; Myers, B.; et al. Chronic Stress Increases Prefrontal Inhibition: A Mechanism for Stress-Induced Prefrontal Dysfunction. Biol. Psychiatry 2016, 80, 754–764.
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2011, 13, 22–37.
- Musazzi, L.; Milanese, M.; Farisello, P.; Zappettini, S.; Tardito, D.; Barbiero, V.S.; Bonifacino, T.; Mallei, A.; Baldelli, P.; Racagni, G.; et al. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: The dampening action of antidepressants. PLoS ONE 2010, 5, e8566.
- Yuen, E.Y.; Wei, J.; Liu, W.; Zhong, P.; Li, X.; Yan, Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 2012, 73, 962–977.
- Segovia, G.; del Arco, A.; Mora, F. Environmental enrichment, prefrontal cortex, stress, and aging of the brain. J. Neural Transm. 2009, 116, 1007–1016.
- Xing, B.; Li, Y.-C.; Gao, W.-J. Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res. 2016, 1641, 217–233.
- Arnsten, A.F.T. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410–422.
- Aston-Jones, G.; Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci. 2005, 28, 403–450.
- Birnbaum, S.; Gobeske, K.T.; Auerbach, J.; Taylor, J.R.; Arnsten, A.F.T. A role for norepinephrine in stress-induced cognitive deficits: α-1-adrenoceptor mediation in the prefrontal cortex. Biol. Psychiatry 1999, 46, 1266–1274.
- Ramos, B.P.; Arnsten, A.F.T. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol. Ther. 2007, 113, 523–536.
- Murphy, B.L.; Arnsten, A.F.; Goldman-Rakic, P.S.; Roth, R.H. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc. Natl. Acad. Sci. USA 1996, 93, 1325–1329.
- Mizoguchi, K.; Yuzurihara, M.; Ishige, A.; Sasaki, H.; Chui, D.-H.; Tabira, T. Chronic Stress Induces Impairment of Spatial Working Memory Because of Prefrontal Dopaminergic Dysfunction. J. Neurosci. 2000, 20, 1568–1574.
- Lupien, S.J.; Maheu, F.; Tu, M.; Fiocco, A.; Schramek, T.E. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007, 65, 209–237.
- Chaouloff, F.; Groc, L. Temporal modulation of hippocampal excitatory transmission by corticosteroids and stress. Front. Neuroendocrinol. 2011, 32, 25–42.
- Gugustea, R.; Jia, Z. Genetic manipulations of AMPA glutamate receptors in hippocampal synaptic plasticity. Neuropharmacology 2021, 194, 108630.
- Kauer, J.A.; Malenka, R.C.; Nicoll, R.A. NMDA application potentiates synaptic transmission in the hippocampus. Nature 1988, 334, 250–252.
- Ortiz, J.B.; Conrad, C.D. The impact from the aftermath of chronic stress on hippocampal structure and function: Is there a recovery? Front. Neuroendocrinol. 2018, 49, 114–123.
- Gould, E.; Tanapat, P. Stress and hippocampal neurogenesis. Biol. Psychiatry 1999, 46, 1472–1479.
- Fuchs, E.; Flügge, G.; Ohl, F.; Lucassen, P.; Vollmann-Honsdorf, G.K.; Michaelis, T. Psychosocial stress, glucocorticoids, and structural alterations in the tree shrew hippocampus. Physiol. Behav. 2001, 73, 285–291.
- Vyas, A.; Pillai, A.G.; Chattarji, S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience 2004, 128, 667–673.
- Conrad, C.D.; Ortiz, J.B.; Judd, J.M. Chronic stress and hippocampal dendritic complexity: Methodological and functional considerations. Physiol. Behav. 2017, 178, 66–81.
- Zhang, X.; Ge, T.T.; Yin, G.; Cui, R.; Zhao, G.; Yang, W. Stress-Induced Functional Alterations in Amygdala: Implications for Neuropsychiatric Diseases. Front. Neurosci. 2018, 12, 367.
- Winklewski, P.J.; Radkowski, M.; Wszedybyl-Winklewska, M.; Demkow, U. Stress Response, Brain Noradrenergic System and Cognition. Adv. Exp. Med. Biol. 2017, 980, 67–74.
- Liu, W.Z.; Zhang, W.H.; Zheng, Z.H.; Zou, J.X.; Liu, X.X.; Huang, S.H.; You, W.J.; He, Y.; Zhang, J.Y.; Wang, X.D.; et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat. Commun. 2020, 11, 2221.
- Vyas, A.; Mitra, R.; Shankaranarayana Rao, B.S.; Chattarji, S. Chronic Stress Induces Contrasting Patterns of Dendritic Remodeling in Hippocampal and Amygdaloid Neurons. J. Neurosci. 2002, 22, 6810–6818.
- Sawamura, T.; Klengel, T.; Armario, A.; Jovanovic, T.; Norrholm, S.D.; Ressler, K.J.; Andero, R. Dexamethasone Treatment Leads to Enhanced Fear Extinction and Dynamic Fkbp5 Regulation in Amygdala. Neuropsychopharmacology 2016, 41, 832–846.
- Rainnie, D.G.; Bergeron, R.; Sajdyk, T.J.; Patil, M.; Gehlert, D.R.; Shekhar, A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J. Neurosci. 2004, 24, 3471–3479.
- Lankford, K.L.; Letourneau, P.C. Evidence that calcium may control neurite outgrowth by regulating the stability of actin filaments. J. Cell Biol. 1989, 109, 1229–1243.
