Sea cucumbers are marine invertebrates that have been used for food, cosmetics, and traditional medicine. Around 100 species of sea cucumbers are harvested for commercial purposes, which have been widely consumed in Asian countries, including China, Indonesia, Japan, Korea, and Malaysia. Traditionally, sea cucumbers are a luxurious and nutritious food, and have been used to cure rheumatism, kidney problems, reproductive disorders, impotence, asthma, joint pain, back pain, hypertension, cuts and burns, wound injuries, and constipation [
1]. In the contemporary market, various products originating from different body parts of the sea cucumbers are available. This mainly includes dry tablets made from the body wall, liquid extract prepared from whole sea cucumbers, and extracts obtained from the skin of sea cucumbers [
2]. Nutritionally, sea cucumbers contain high levels of protein (40–60%), and low levels of lipids (mainly polyunsturated fatty acids (PUFAs)), minerals (e.g., calcium, zinc, iron, and magnesium), and vitamins (e.g., A, B1, B2, and B3) [
3]. Apart from these, sea cucumbers contain a series of bioactive compounds, including triterpene glycosides (saponins), phenolics (flavonoids and phenolic acids), polysaccharides (fucosylated chondroitin sulfate), proteins (collagen and peptides), cerebrosides, and sphingoids, which demonstrate potential antioxidant, anticancer, anti-hypertension, anti-inflammatory, antithrombotic, anti-diabetic, anti-obesity, and antimicrobial activities [
2]. However, biological activities mainly depend on the species, chemical structures, molecular weights, and testing methods. For example, fucosylated chondroitin sulfate of different sea cucumbers (
Stichopus tremulus,
Pearsonothuria graeffei,
Isostichopus badionotus, and
Holothuria vagabunda) exhibited potent anticoagulant activity, which could be associated with the sulfation pattern of the fucose branch [
4]. Moreover, Hossain et al. [
5] suggested that the antioxidant activity of sea cucumber phenolics is mostly linked with the nature of phenolic compounds, sea cucumber body parts, pre-treatment, and the assays used to determine the activity.
2. Bioactive Compounds of Sea Cucumbers and Their Antioxidant Activity
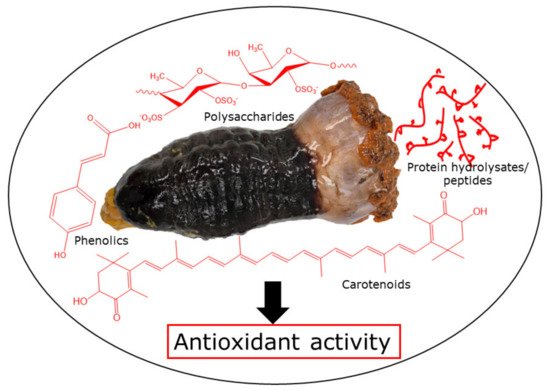
Sea cucumbers are a highly marketable echinoderm, which contains numerous bioactive compounds. These include proteins (collagen and peptides), polysaccharides, saponins, carotenoids, and phenolics with multiple biological and pharmacological properties, mainly antioxidant activity [
2,
7]. Antioxidants are substances that scavenge free radicals and hence prevent oxidation. The main mechanisms involved are hydrogen atom transfer (HAT), single electron transfer (SET), metal chelation, and reducing power. Therefore, the effectiveness of antioxidants in a specific medium is mainly dependent on the number and arrangement of the hydroxyl groups in the molecules of interest [
8]. For example, phenolic antioxidants can donate hydrogen atoms from the hydroxyl groups to lipid radicals in order to neutralize the oxidation reaction, but the phenoxyl radicals that are produced are resonance stabilized and are therefore not involved in further oxidation, thus breaking the cycle of the generation of new radicals. Antioxidants with specific structures can also chelate metal ions (e.g., ferrous and copper), where metal ions can no longer act as an initiator of lipid oxidation due to the formation of a complex between the metal ions and antioxidants. Besides, synergistic effects can be observed among various antioxidants such as phenolics, α-tocopherol, β-carotene, and ascorbic acid [
9,
10]. Numerous techniques are available for determining antioxidant activity, including radical scavenging assays that include SET (e.g., ferric-reducing antioxidant power (FRAP), Trolox equivalent antioxidant capacity (TEAC), and the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay) and HAT (e.g., total radical-trapping antioxidant parameter (TRAP) and oxygen radical absorbance capacity (ORAC)) mechanisms [
11,
12]. However, each assay has a different mechanism of action, thus providing varied results for the antioxidant potential of the same sample [
13]. On the other hand, due to lipid oxidation, the quality attributes of food, including flavor, color, and texture, deteriorate, which ultimately decreases the shelf life and nutritional value of food. Thus, antioxidants are widely used to control the rate and extent of lipid oxidation in foods. One of the main assays to measure the degree of lipid oxidation is the thiobarbituric acid (TBA) test that measures the TBA reactive substances (TBARS), which are then used to determine the secondary oxidation products, mainly the aldehydes, from among the others that are believed to produce rancid flavors and aromas [
14]. Autoxidation is one of the main pathways of lipid oxidation in which PUFAs are involved in a free radical chain reaction under heat, light, or metal ions. Synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), tert-butylhydroquinone (TBHQ), and propyl gallate (PG) have been used as antioxidants in foods to prevent oxidation and off-flavor development. Nevertheless, due to the carcinogenic and toxicity characteristics of some synthetic antioxidants, researchers have shown much attention towards natural antioxidants [
15,
16]. For instance, sea cucumbers and their by-products are a good source of phenolic acids and flavonoids, which show strong antioxidant activity. The specific bioactive compounds in most common sea cucumbers and their antioxidant activities are detailed below (
Figure 1).
Figure 1. Sea cucumber antioxidants.
2.1. Antioxidant Potential of Sea Cucumber Phenolics and Their Beneficial Effects on Human Health
Phenolic compounds are secondary metabolites that contain one or more aromatic rings and hydroxyl groups. Moreover, phenolic compounds play a key role in protecting plants by engaging in defense mechanisms against ultraviolet radiation, herbivory, and pathogen attacks. Phenolics are also involved in the plants for growth regulation and are responsible for the color, flavor, bitterness, and astringency of foods. Plant phenolics are mainly derived from phenylalanine and, in some cases, tyrosine. The formation of
trans-cinnamic acid from phenylalanine is catalyzed by phenylalanine ammonia-lyase (PAL), whereas
p-hydroxycinnamic acid from tyrosine is catalyzed by tyrosine ammonia-lyase (TAL) [
17]. Phenolics can be categorized into different groups, namely phenolic acids, flavonoids, tannins, stilbenes, lignans, and coumarins [
18]. Due to their antioxidant, antimicrobial, and coloring properties, phenolic compounds have received significant attention from several industries, especially from the food, pharmaceutical, cosmetics, packaging, and textile industries. In the food industry, phenolics are used as preservatives to inhibit the oxidation process and microbial growth of food products.
Plant- and marine-based phenolic compounds are receiving increased attention due to their potential health benefits and multiple biological activities. Most of the phenolics have so far been researched from the terrestrial environment, but less attention has been paid to the marine environment, even though it provides many healthy foods due to its biodiversity. Sea cucumbers are one of the marine invertebrates that serves as a possible source of phenolic compounds with strong antioxidant activity. This could be due to the absorption of phenolics from phytoplankton, the primary food source for sea cucumbers. Phytoplankton is a rich source of phenolic compounds, including phenolic acids, flavonoids, and tannins [
2,
19]. For example, Ceesay et al. [
20] reported that sea cucumbers contain catechins and flavonols as they feed mainly on seaweeds, rich sources of flavonoids. Various species of sea cucumbers have different levels of phenolic compounds and varied antioxidant activities. This might be due to the different geographic locations, food habits, and harvesting times. Therefore, suspension-feeding species, such as
Cucumaria frondosa, may have more phenolics when compared to the deposit-feeding species.
It has been reported that the different body parts of sea cucumbers, such as body wall, tentacles/flower, and viscera, contain a significant amount of phenolics with strong antioxidant activity. For example, Althunibat et al. [
21] compared the antioxidant activity of three Malaysian sea cucumber species (
Holothuria leucospilota,
Holothuria scabra, and
Stichopus chloronotus) without viscera, and reported that the extracts of
H. leucospilota had higher total phenolic contents (TPC, 9.7 mg gallic acid equivalents (GAE)/g), but
H. scabra contained a lower amount of TPC (1.53 mg GAE/g).
S. chloronotus extracts showed a higher DPPH radical-scavenging capacity (80.58%) compared to the
H. scabra (77.46%) and
H. leucospilota (64.03%) extracts. Likewise, methanol extracts of
H. scabra were found to be a good source of phenolics (30.52 mg GAE/g), dominated by 3- and 4-hydroxybenzaldehyde [
22]. Wulandari et al. [
23] cultured
Holothuria scabra in an open pond system and found that antioxidant activity such as ABTS and hydroxyl radical-scavenging activities as well as ferric-reducing antioxidant power (FRAP) were related to the total flavonoid content (TFC). Besides, TPC and TFC were determined in the body wall of
H. leucospilota, which contained 2,4-bis(1,1-dimethylethyl)-phenol [
20]. Pre-treatments also affect the content of sea cucumber phenolic and their antioxidant activities. For example, free, esterified, and insoluble-bound phenolics from the body wall and internal organs of Atlantic sea cucumber (
Cucumaria frondosa) were determined using high-pressure processing (HPP) pre-treatment [
5,
24]. Results demonstrated that HPP significantly improved the TPC, TFC, and antioxidant activity. TPC varied between 3.05 and 3.98 mg GAE/g for the body wall and 2.32–3.02 mg GAE/g for internal organs, while the TFC was 1.22–1.55 mg catechin equivalents (CE)/g and 1.01–1.24 mg CE/g for body wall and internal organs, respectively. Additionally, phenolic extracts obtained from the body wall and internal organs exhibited strong antioxidant activity in terms of DPPH, ABTS, and hydroxyl radical scavenging as well as metal chelation activities, which showed a strong positive correlation with TPC and TFC. Especially, TFC had a strong correlation with antioxidant activity, suggesting that sea cucumber phenolics are mostly flavonoids, which are responsible for the antioxidant effect. On the other hand, the antioxidant activity, TPC, and TFC were determined in extracts from different body parts (digestive tract, gonad, muscle, and respiratory apparatus) of
C. frondosa [
25]. The TPC varied from 0.22 to 2.36 mg GAE/g, while TFC ranged from 0.029 to 0.59 mg rutin equivalents (RE)/g and the oxygen radical absorbance capacity (ORAC) varied from 140 to 800 µmol Trolox equivalents (TE)/g. A higher TPC was also observed in the digestive tract when considering acetonitrile-rich fractions and ethyl acetate extracts, while the maximum TFC was obtained from the gonads using water-rich and acetonitrile-rich fractions. Similarly, Mamelona and Pelletier [
26] determined the antioxidant activity (ORAC) of the viscera of
C. frondosa using pressure liquid extraction (PLE) and observed that the ethanol extracts had higher ORAC values when compared to methanol, isopropanol, and water extracts at 60 °C extraction. Additionally, PLE allowed better extraction of TPC, total carotenoids, and α-tocopherol using ethanol followed by isopropanol, methanol, and water. In another study, the antioxidant property of fresh and processed
C. frondosa with/without internal organs was evaluated [
19]. The processed (rehydrated) samples, mainly those with internal organs, exhibited higher ORAC and DPPPH radical-scavenging activity, whereas fresh samples contained a significant amount of phenolics when compared to their rehydrated counterparts. However, Ridhowati et al. [
27] reported that dried
Stichopus variegatus contained a higher content of TPC (10.55–10.9 mg GAE/g) with strong DPPH radical-scavenging activity. Similarly, Husni et al. [
28] stated that the TPC of
Apostichopus japonicus body wall extract had a good correlation with antioxidant activity than the TFC, suggesting that phenolic compounds play an important role in exhibiting antioxidant activity.
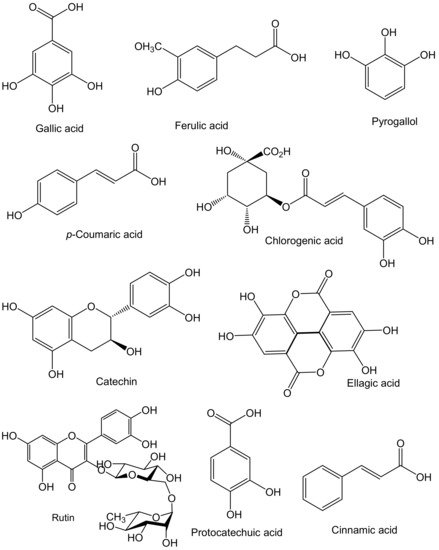
Sea cucumber phenolics are mainly phenolic acids and flavonoids. The most common phenolic compounds found in sea cucumbers are chlorogenic acid, gallic acid, p-coumaric acid, protocatechuic acid, ferulic acid, ellagic acid, cinnamic acid, catechin, rutin, quercetin, and pyrogallol (Figure 2).
Figure 2. Major phenolic compounds found in sea cucumbers.
2.2. Antioxidant Potential of Protein Hydrolysates and Peptides and Their Health Benefits
Marine products and by-products are protein-rich and can be used to prepare protein hydrolysates, collagens, or peptides. The most common ways of producing protein hydrolysates include enzymatic (Flavourzyme, Alcalase, Protamex, papain, trypsin, chymotrypsin, and pepsin, among others), non-enzymatic (high-pressure processing, ultrasound, and supercritical fluids), organic solvents, and fermentation methods [
41,
42]. Among them, enzymatic procedures have received growing attention due to their higher efficiency, green nature, and lesser destruction than other techniques in order to produce value-added products for disease risk reduction and health promotion. In particular, microbial proteases, including Flavourzyme, Alcalase, and Corolase are favorable in industrial use due to their promising operational conditions [
43]. However, the functionality of protein hydrolysates/peptides is mainly dependent on their amino acid compositions and sequences, molecular weight, and hydrophobicity/hydrophilicity, among others. Generally, bioactive peptides contain 3–20 amino acid units and show antioxidant activity [
44]. Notably, the antioxidant activity of the bioactive peptides can improve in the presence of amino acids such as tyrosine, phenylalanine, proline, glutamic acid, histidine, and arginine. For example, proline is very common in collagen, shielding cells from the oxidation induced by free radicals [
45].
The physicochemical and antioxidant properties of the protein hydrolysates of
C. frondosa and its processing discards were evaluated using Alcalase, Flavourzyme, and Corolase as well as their combination [
43]. The hydrolysates prepared with combination of enzymes were predominant in glutamic acid and displayed the highest radical-scavenging activity against ABTS and DPPH radicals as well as metal-chelation activity. In addition, hydrolysates were able to inhibit TBARS production in a meat model system and beta-carotene bleaching in an oil-in-water emulsion. They also noted that the level of free amino acids after hydrolysis, the degree of hydrolysis, amino acid sequence, and molecular weight played the main role in rendering radical-scavenging activity. Similarly, Yan et al. [
46] prepared enzymatic hydrolysates from
C. frondosa viscera using Alcalase, Flavourzyme, Neutrase, trypsin, papain, and bromelain and observed that Alcalase, Flavourzyme, and trypsin were major enzymes that resulted in strong antioxidant activity, possibly related to a high amount of hydrophobic amino acids in the hydrolysates. The choice of proteases in the preparation of protein hydrolysates plays a significant role in the resultant antioxidant potency and bioactivity of peptides. For example, Alcalase-produced protein hydrolysates obtained from
C. frondosa exhibited up to 35% higher in vitro antioxidant activity (e.g., DPPH radical, hydroxyl radical, and superoxide radical anion-scavenging properties) than the trypsin-produced hydrolysates, suggesting that the amino acid composition and structural conformation of the peptides played main roles in determining antioxidant activity [
47]. This is because trypsin-produced peptide fractions were not the same as Alcalase-produced peptide fractions, mainly when the sizes of the peptides were small (≤10 kDa). Moreover, in silico docking for in vivo function prediction demonstrated a better inhibitory activity in myeloperoxidase (a marker of in vivo oxidative stress) by Alcalase than by trypsin. Likewise, protein hydrolysates obtained from Atlantic sea cucumber viscera showed antioxidant activities in lipid oxidation tests and the ORAC assay, which could be related to the releasing of antioxidative peptides upon hydrolysis [
48]. The findings of Wang et al. [
49] suggest that
C. frondosa internal organs hydrolysates have the potential to show anti-diabetic activity through insulin resistance and lipid metabolism syndromes. Besides, enzymatic hydrolysates prepared from
C. frondosa by-products (aquapharyngeal bulb and internal organs) using nine different proteases, including Alcalase, bromelain, Flavourzyme, fungal protease, neutral protease, papain, peptidase AM (
Aspergillus melleus), peptidase AO (
Aspergillus oryzae), and Protamex, were tested against Herpes Simplex virus 1 (HSV-1) [
50]. Results suggested that papain was the most efficient enzyme in demonstrating antiviral activities. Lin et al. [
51] reported the anti-aging effect of sea cucumber (
C. frondosa) hydrolysates, which was mainly linked to the low-molecular-weight (~<3 kDa) peptides. The anti-aging mechanism could be related to the up-regulated Klotho expression, down-regulated acetylcholinesterase activity, increased SOD and GSH-Px activities, and lipid and protein oxidation inhibition.
2.3. Antioxidant Potential of Sea Cucumber Polysaccharides
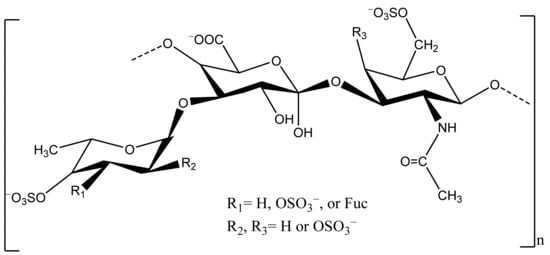
Sea cucumber, mainly the body wall, is a good source of polysaccharides, including sulfated polysaccharides (fucosylated chondroitin sulfate, FCS) and fucan (Figure 3).
Figure 3. Chemical structure of FCS of sea cucumber.
The chain conformation of polysaccharides mainly affects their antioxidant activity. In particular, the antioxidant activity of sulfated polysaccharides is associated with the molecular weight, degree of sulfation, type of main sugars, and glycosidic bonds. Hence, the antioxidant activity of sea cucumber polysaccharides is not related to a single factor but rather a combination of several factors. For example, Liu et al. [
61] reported that the polysaccharides of
Apostichopus japonicus were mainly composed of glucosamine, glucuronic acid, galactosamine, mannose, galactose, glucose, and fucose, which showed potent hydroxyl, DPPH, and superoxide radical-scavenging activities as well as reducing power. These could be due to the ability of free radicals to abstract anomeric hydrogen from polysaccharides [
62]. Similarly, polysaccharides obtained from
Phyllophorus proteus exhibited hydroxyl, DPPH, ABTS, and superoxide radical-scavenging activities, which may closely be linked to their structural features, including monosaccharide compositions and contents of their sulfate and carboxyl groups [
63]. In particular, sulfate and carboxyl groups are extremely nucleophilic and may chelate metal ions (e.g., Cu
2+ and Fe
2+) and show hydroxyl radical-scavenging activity. Moreover, the sulfate group in sulfated polysaccharides could initiate superoxide radical-scavenging activity due to its electron-donating substituents in a saccharide ring [
64]. However, Gao et al. [
65] claimed that
Holothuria fuscopunctata polysaccharides (fucan sulfate) had potent antioxidant activity for superoxide radicals, while exhibiting almost no scavenging effect for hydroxyl, DPPH, and ABTS radicals, which is related to the structural characteristics of sulfated polysaccharides. Moreover, Li et al. [
66] found that
Stichopus chloronotus fucoidan mainly consists of L-fucose and sulfate esters, which demonstrate significant inhibition of lipid peroxidation and immunoregulatory properties. The sulfate content, sulfate patterns, and molecular weight of fucoidan especially affect the inhibitory activities of superoxide radicals. Likewise, ultrasound treatment was found to slightly improve the antioxidant activity (DPPH radical-scavenging activity and ORAC) of fucoidan obtained from
Isostichopus badionotus [
67] which could be related to its lower molecular weight. Furthermore, Li et al. [
68] stated that the sulfated polysaccharides of
Holothuria fuscogliva show strong hydroxyl and superoxide radical scavenging activities as well as anticoagulant properties. On the other hand, the sulfation patterns of fucose branches of FCS obtained from Stichopus chloronotus,
Apostichopus japonicus, and
Acaudina molpadioidea were 4-
O-, 2,4-di-
O, and 3,4-di-
O-sulfation, respectively, which inhibited DPPH and nitric oxide radicals as well as lipid peroxidation. The inhibitory activity may be affected by the sulfation patterns of the fucose branches. In this regards, 4-
O sulfation residues exhibit the strongest antioxidant properties, while the opposite scenario is seen for 3,4-
O-sulfated fucose residues [
69]. Yu et al. [
70] prepared fucoidan from
Thelenota ananas and found a significant inhibitory effect of polysaccharides for superoxide radicals, closely related to its sulfate groups. Similarly, FCS isolated from
Acaudina molpadioidea and
Holothuria nobilis showed moderate antioxidant properties against hydroxyl, DPPH, and superoxide radicals in a dose-dependent manner. The presence of sulfate groups in polysaccharides with the ability to bind metal ions was found to be responsible [
71]. In addition, sea cucumber (
Apostichopus japonicus) gonadal polysaccharide exhibited DPPH and hydroxyl radical-scavenging activities as well as reducing power. The activity was greater in the presence of a higher content of sulfate groups and maintaining a lower molecular weight [
72]. Besides, Qi et al. [
73] suggested that sea cucumber processing liquor is mainly composed of mannose, glucose, and fucose, which showed strong DPPH, hydroxyl, and superoxide radical anion-scavenging activities. Moreover, in an in vivo model, these polysaccharides were able to increase the activity of catalase and SOD.
2.4. Antioxidant Potential of Carotenoids and Physiological Effects of PUFAs
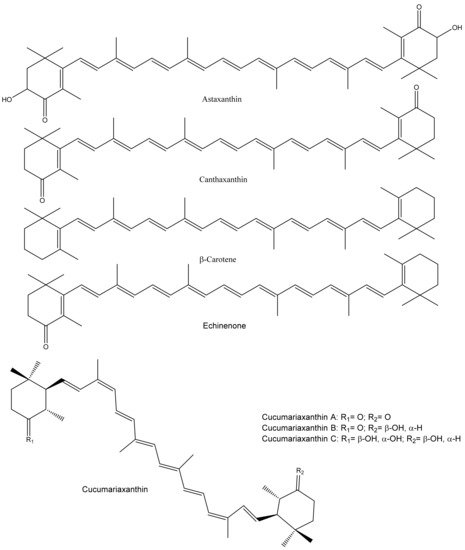
Marine animals, including sea cucumbers, are a good source of carotenoids that show structural diversity. Sea cucumbers, mainly their gonads and aquapharyngeal bulb/tentacles, contain various carotenoids (Figure 4).
Figure 4. Major carotenoids found in sea cucumbers.
The distinctive color of their internal organs is due to carotenoids that contain a series of conjugated C=C. Sea cucumbers, mostly passive feeder species, use algal or other carotenoid-rich materials as their primary food source, resulting in carotenoid accumulation in their tissues. The major carotenoids in sea cucumbers are astaxanthin and canthaxanthin, and their composition varies with species, geographical location, and body part. For example, Matsuno and Tsushima [
74] investigated the carotenoid composition of seven sea cucumber species (
Stichopus japonicus,
Holothuria moebi,
Holothuria pervicax,
Holothuria leucospilota,
Cucumaria echinata,
Cucumaria japonica, and
Pentacta australis), and β-echinenone, β-carotene, phoenicoxanthin, canthaxanthin, and astaxanthin were found in all of them. However, cucumariaxanthin A, B, and C were only present in
C. echinata,
C. japonica, and
P. australis. Furthermore, Tsushima et al. [
75] identified carotenoids (5,6,5′,6′-tetrahydro-carotenoids with 9Z,9′Z configurations) termed as cucumariaxanthins A, B, and C from
Cucumaria japonica gonads, where cucumariaxanthin C showed antiviral activity on Epstein-Barr virus activation. Similarly, Maoka et al. [
76] identified a new carotenoid (9Z,9′Z-tetrahydroastaxanthin) along with echinenone, canthaxanthin, β-carotene, astaxanthin, adonirubin, and cucumariaxanthin A from
Plesiocolochirus minutusalong. Recently, 14 carotenoids were identified from the
Cucumaria frondosa japonica Semper using supercritical CO
2 extraction, where cucumariaxanthin and canthaxanthin were abundant [
77]. In addition, the composition of fatty acids and carotenoids were analyzed from 12 sea cucumbers, and their effect on cancer cells was tested [
78]. It was found that PUFAs, mainly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and carotenoids significantly contributed to the cytotoxic activity against cervical (HeLa), colon (WiDR), and breast (T47D and MCF-7) cancer cells. However, little is known about the antioxidant activity of sea cucumber carotenoids and their detailed mechanisms. The antioxidant activity of these carotenoids could be related to the scavenging of ROS, mainly singlet oxygen (
1O
2) and peroxyl radicals, due to their special chemical features (conjugated C=C) [
79].
2.5. Antioxidant Potential of Other Bioactive Compounds of Sea Cucumber
Apart from phenolics, protein hydrolysates/peptides, polysaccharides, and carotenoids, other bioactive compounds such as the saponins and cerebrosides of the sea cucumber also exhibit potential antioxidant activity. For example, the antioxidant and cytotoxic activities of organic and aqueous extracts of
Stichopus horrens and
Holothuria edulis were investigated and both extracts showed strong antioxidant activity against DPPH and linoleate (β-carotene bleaching assay) radicals. Meanwhile the organic extract showed higher cytotoxic effects against A549 (lung cancer) and TE1 (esophageal cancer) cells [
88]. Moreover, four sulfated holostan-type triterpene glycosides (echinoside B 12-
O-methyl ether, echinoside B, 24-dehydroechinoside B, and holothurin B) were isolated from the Saudi Red Sea cucumber
Holothuria atra, which demonstrated antioxidant activity (reducing power and DPPH radical-scavenging activity) and cytotoxic effects against Ehrlich ascites carcinoma cells [
89]. Furthermore, processing methods have a significant effect on antioxidant and physicochemical properties of sea cucumber products. For instance, sea cucumber powder (
Holothuria scabra) was made using microwave heating, smoking, and steaming; the microwave treatment rendered a stronger DPPH radical-scavenging activity than the other two methods, possibly related to the heating via direct interaction with microwaved materials [
90]. The antioxidant activity of microwave-treated powder could be linked to the presence of phenolics, steroids, and alkaloids. In addition, 21 Indonesian sea cucumber extracts were tested for their biological activities;
H. atra and
S. vastus extracts showed strong antioxidant (IC
50 = 14.22 µg/µL) and antiviral activities, respectively [
91]. Similarly, Rasyid et al. [
92] analyzed the ABTS radical-scavenging activity of five Indonesian sea cucumber extracts, where
H. leucospilota,
H. lessoni, and
Stichopus quadrifasciatus extracts had the strongest scavenging activity. In another study, Wulandari et al. [
93] stated that
H. scabra cultivated in the pond for 12 months demonstrated strong antioxidant and antibacterial activities. However, Wu et al. [
94] determined the effect of
Acaudina molpadioides cerebrosides on the tert-butyl hydroperoxide and hydrogen peroxide-induced oxidative damage in PC12 cells. The sea cucumber cerebrosides had a positive effect against oxidative damage by inhibiting mitochondria-mediated apoptosis, which may be related to their antioxidant effect.