Mitral regurgitation is the second-most frequent valvular heart disease in Europe after degenerative aortic stenosis. The mitral valve is a complex anatomical structure whose physiological functioning relies on the biomechanical properties and structural integrity of its components. Their compromise can lead to mitral valve dysfunction. Assessing its morphology can reveal various normal and abnormal features, which can be associated with deteriorating clinical outcomes. Transthoracic echocardiography is the first diagnosis approach that should be performed to assess the valve disfunction, giving a complete morphological description of the structures, the regurgitant mechanism, and etiology.
- mitral regurgitation
- mitral valve
- echocardiography
1. Introduction
2. Etiology and Regurgitant Mechanisms
2.1. Primary Mitral Regurgitation
2.2. Secondary Mitral Regurgitation
2.3. Carpentier Classification
MR can be classified according to the Carpentier classification (Table 1), which facilitates the understanding of the etiology as well as therapeutic approach [9].
| Carpentier’s Classification |
Leaflets Motion | Anatomical Session | Etiologies |
|---|---|---|---|
| Type I | Normal. | Leaflet perforation. Annular dilatation. |
Degenerative (annular calcification), infectious endocarditis, inflammatory, congenital cleft defect. |
| Type II | Excesive. | Chordal rupture. Chordal elongation. Papillary muscle rupture. |
Degenerative (Barlow’s disease), congenital, infectious, ischemic. |
| Type IIIa | Restricted in both systole and diastole. | Commisural or chordal fussion. Leaflet thickening. Leaflet calcification. |
Rheumatic, inflammatory, radiation, drugs. |
| Type IIIb | Restricted in systole. | Ventricular dilatation. Chordal thickening or shortening. |
Ischemic and non-ischemic. |
3. Severity Assessment in Practice
MR severity assessment is a challenging process in which a multimodality evaluation, integrating quantitative, semiquantitative and qualitative methods, as well as a detailed evaluation of the morphology and function of both LV and LA is the key [4]. There is a strong correlation between the hemodynamic status of the patient and the quantification of the MR. Quantification variability due to the regurgitation dependence on the cardiac afterload and preload makes it important to choose the right clinical time to perform a systematic and accurate echocardiographic assessment in a dynamic scenario [10][11].
3.1. Quantitative Parameters
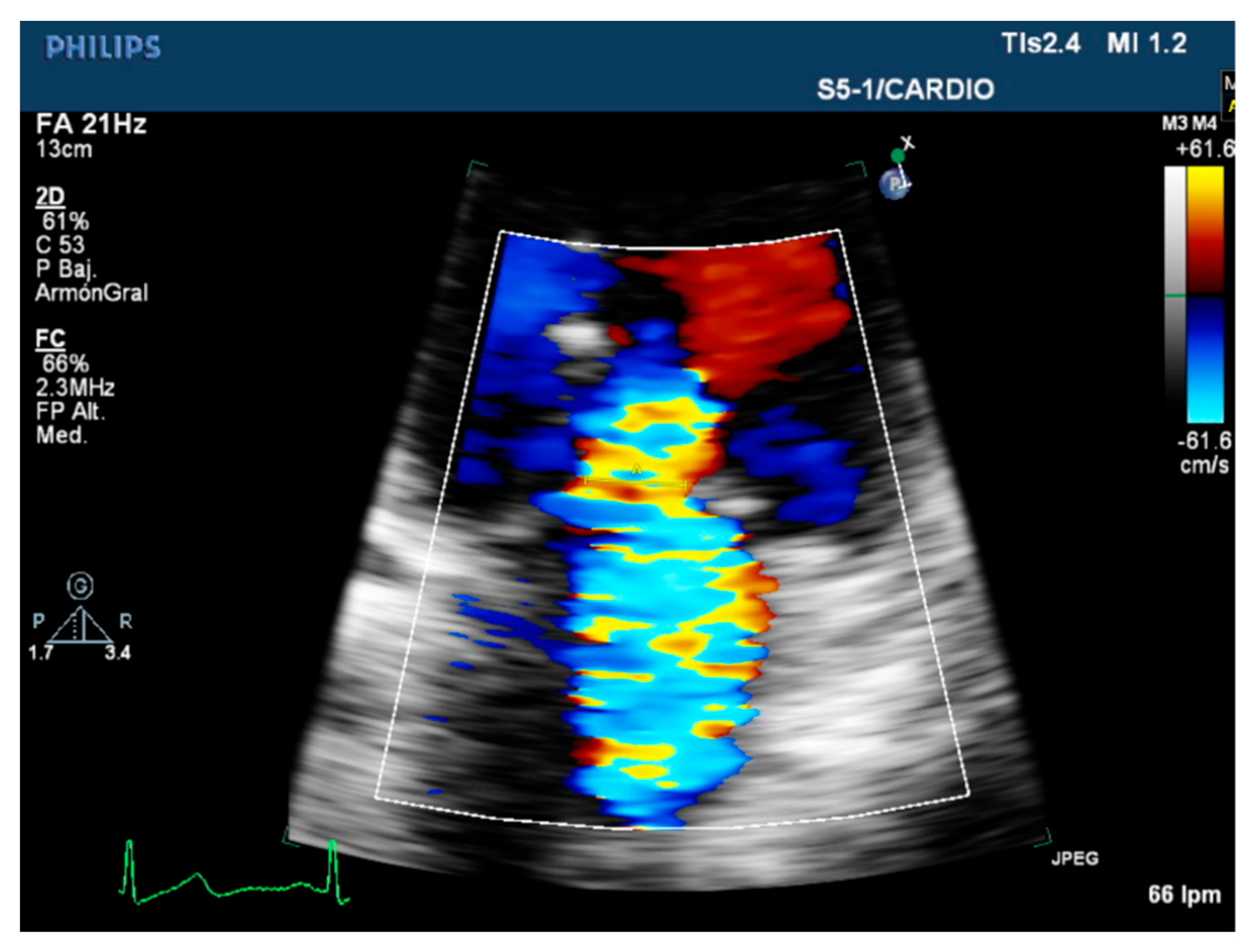
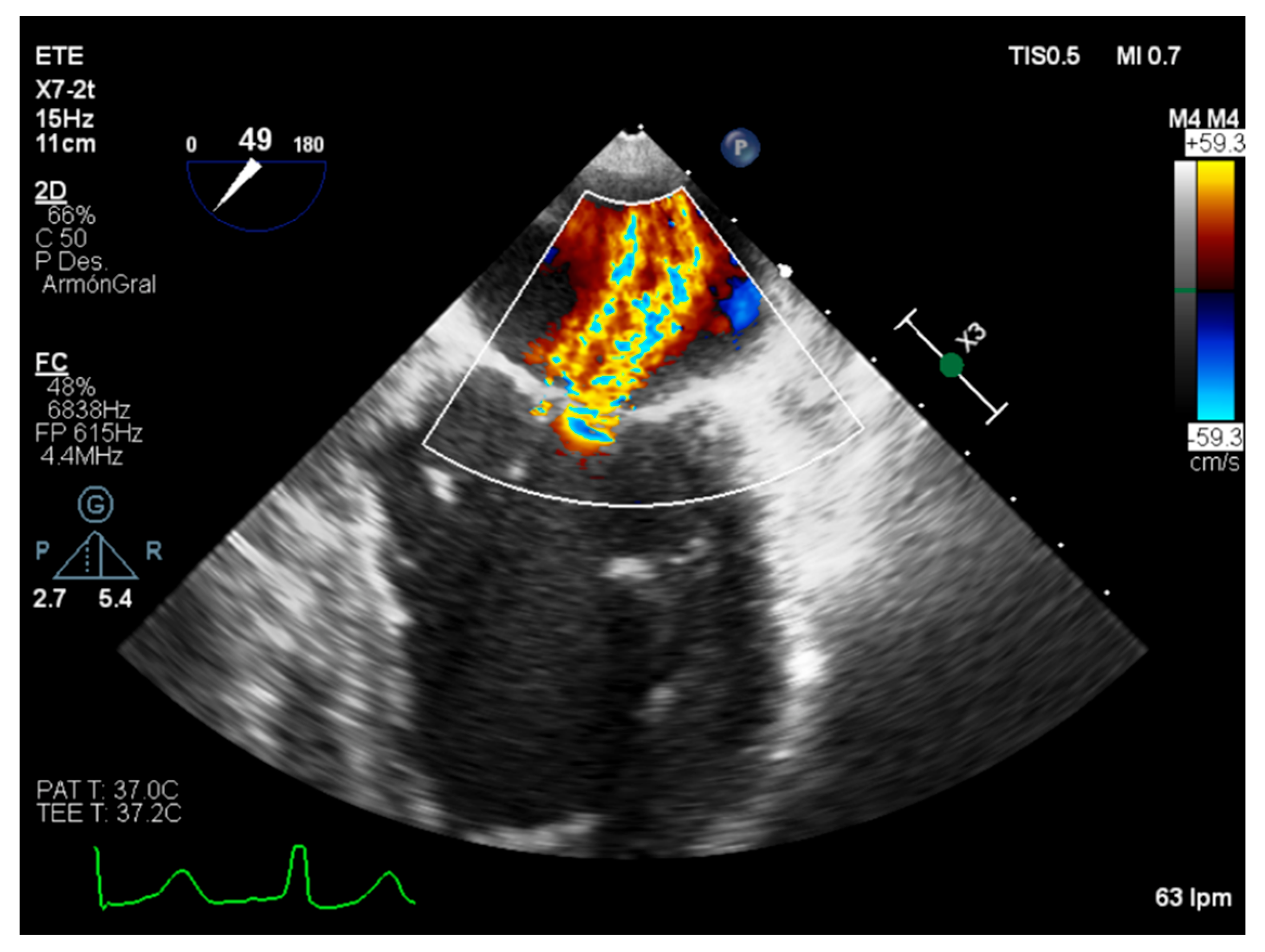
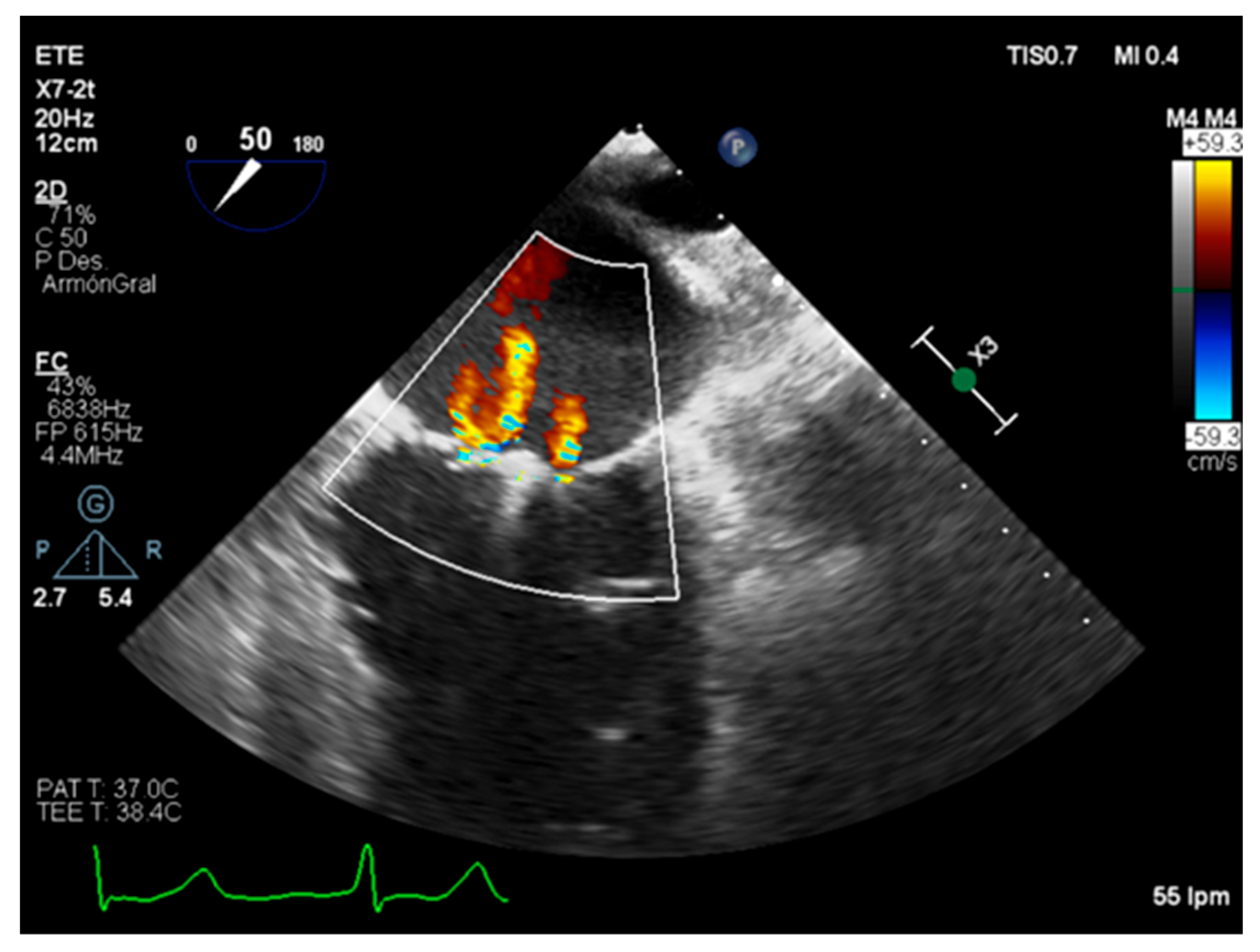
3.2. Semiquantitative and Qualitative Parameters
3.3. Role of Stress Echocardiography
4. Assessment of Suitability of Valvular Repair
5. Role of Intraoperative Echocardiography
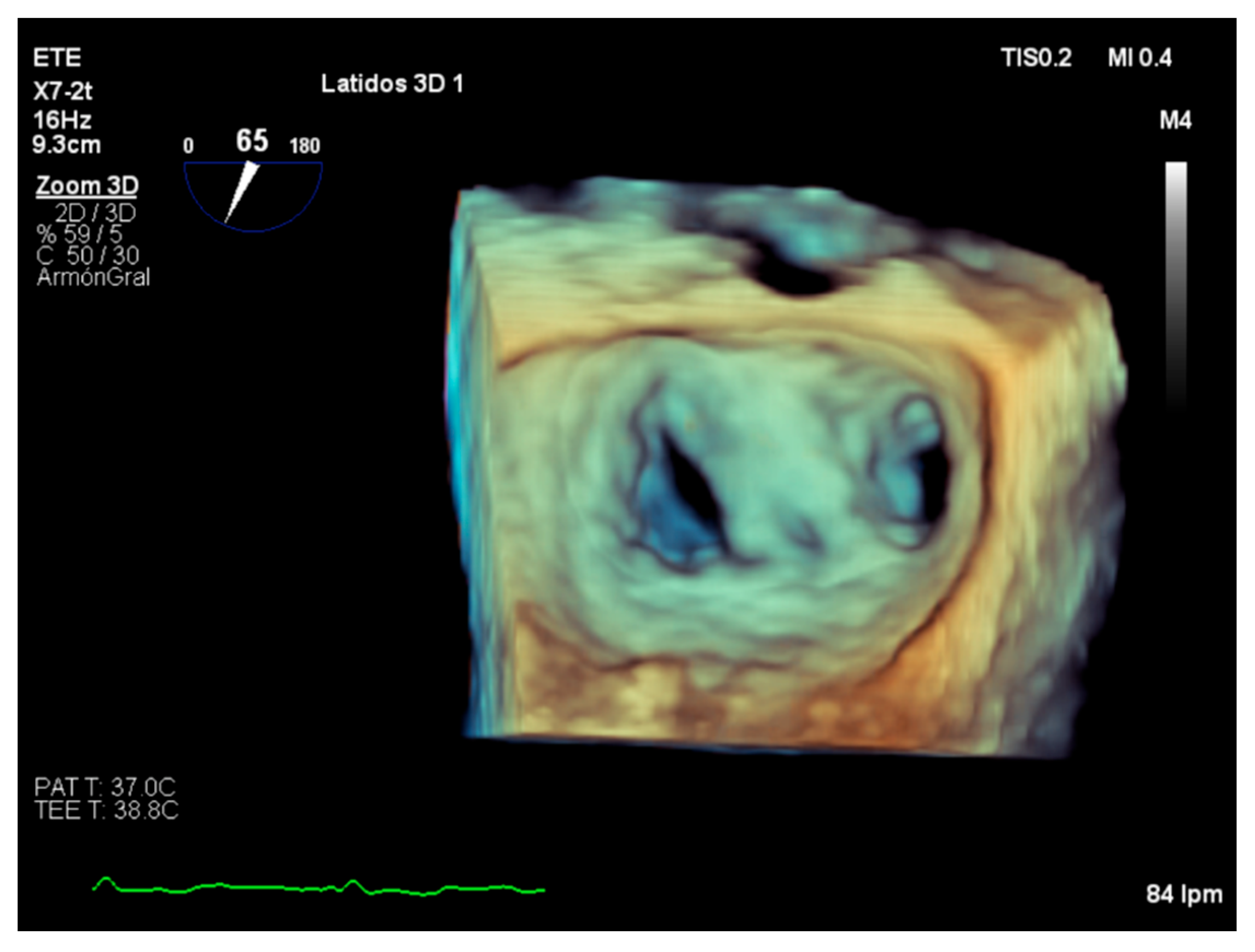
7. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12071717
References
- Enriquez-Sarano, M.; Akins, C.; Vahanian, A. Mitral regurgitation. Lancet 2009, 373, 1382–1394.
- Dal-Bianco, J.; Beaudoin, J.; Handschumacher, M.; Levine, R. Basic Mechanisms of Mitral Regurgitation. Can. J. Cardiol. 2014, 30, 971–981.
- Bonow, R.O.; O’Gara, P.T.; Adams, D.H.; Badhwar, V.; Bavaria, J.E.; Elmariah, S.; Hung, J.W.; Lindenfeld, J.; Morris, A.A.; Satpathy, R.; et al. 2020 Focused Update of the 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation. J. Am. Coll. Cardiol. 2020, 75, 2236–2270.
- Dulgheru, R.; Bruls, S.; Lancellotti, P. How I look at the regurgitant mitral valve—A stepwise echocardiographic assessment. Eur. Heart J.-Cardiovasc. Imaging 2020, 22, 491–493.
- El Sabbagh, A.; Reddy, Y.; Nishimura, R. Mitral Valve Regurgitation in the Contemporary Era. JACC Cardiovasc. Imaging 2018, 11, 628–643.
- Zeng, X.; Tan, T.; Dudzinski, D.; Hung, J. Echocardiography of the Mitral Valve. Prog. Cardiovasc. Dis. 2014, 57, 55–73.
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, 43, 561–632.
- Ingraham, B.; Chareonthaitawee, P.; Reddy, Y. Exercise-Induced Left Bundle Branch Block Resulting in Severe Mitral Regurgitation. JACC Case Rep. 2021, 3, 1287–1290.
- Grayburn, P.; Thomas, J. Basic Principles of the Echocardiographic Evaluation of Mitral Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 843–853.
- Stevenson, L.; Brunken, R.; Belil, D.; Grover-McKay, M.; Schwaiger, M.; Schelbert, H.; Tillisch, J. Afterload reduction with vasodilators and diuretics decreases mitral regurgitation during upright exercise in advanced heart failure. J. Am. Coll. Cardiol. 1990, 15, 174–180.
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.; Edvardsen, T.; Pierard, L.; Badano, L.; Zamorano, J. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2013, 14, 611–644.
- Quéré, J.; Tribouilloy, C.; Enriquez-Sarano, M. Vena contracta width measurement: Theoretic basis and usefulness in the assessment of valvular regurgitation severity. Curr. Cardiol. Rep. 2003, 5, 110–115.
- Grayburn, P.; Sannino, A.; Packer, M. Proportionate and Disproportionate Functional Mitral Regurgitation: A New Conceptual Framework That Reconciles the Results of the MITRA-FR and COAPT Trials. JACC Cardiovasc. Imaging 2019, 12, 353–362.
- Robinson, S.; Ring, L.; Augustine, D.; Rekhraj, S.; Oxborough, D.; Harkness, A.; Lancellotti, P.; Rana, B. The assessment of mitral valve disease: A guideline from the British Society of Echocardiography. Echo Res. Pract. 2021, 8, G87–G136.
- Buck, T.; Plicht, B. Real-Time Three-Dimensional Echocardiographic Assessment of Severity of Mitral Regurgitation Using Proximal Isovelocity Surface Area and Vena Contracta Area Method. Lessons We Learned and Clinical Implications. Curr. Cardiovasc. Imaging Rep. 2015, 8, 38.
- Asch, F.M.; Grayburn, P.A.; Siegel, R.J.; Kar, S.; Lim, D.S.; Zaroff, J.G.; Mishell, J.M.; Whisenant, B.; Mack, M.J.; Lindenfeld, J.; et al. Echocardiographic Outcomes After Transcatheter Leaflet Approximation in Patients with Secondary Mitral Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 2969–2979.
- Obadia, J.-F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306.
- Tribouilloy, C.; Shen, W.F.; Rey, J.L.; Adam, M.C.; Lesbre, J.P. Mitral to aortic velocity-time integral ratio. A non-geometric pulsed-Doppler regurgitant index in isolated pure mitral regurgitation. Eur. Heart J. 1994, 15, 1335–1339.
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 1191–1229.
- Magne, J.; Lancellotti, P.; Piérard, L.A. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation 2010, 122, 33–41.
- Pierard, L.; Dulgheru, R.; Magne, J.; Lancellotti, P. Dynamic ischaemic mitral regurgitation and the role of stress echocardiography. J. Cardiovasc. Echogr. 2013, 23, 10–17.
- Suzuki, T.; Izumo, M.; Suzuki, K.; Koto, D.; Tsukahara, M.; Teramoto, K.; Sato, Y.; Watanabe, M.; Mizukoshi, K.; Kamijima, R.; et al. Prognostic value of exercise stress echocardiography in patients with secondary mitral regurgitation: A long-term follow-up study. J. Echocardiogr. 2018, 17, 147–156.
- Jung, J.C.; Jang, M.J.; Hwang, H.Y. Meta-analysis comparing mitral valve repair versus replacement for degenerative mitral regurgitation across all ages. Am. J. Cardiol. 2019, 123, 446–453.
- Omran, A.; Woo, A.; David, T.; Feindel, C.; Rakowski, H.; Siu, S. Intraoperative transesophageal echocardiography accurately predicts mitral valve anatomy and suitability for repair. J. Am. Soc. Echocardiogr. 2002, 15, 950–957.
- Thaden, J.J.; Malouf, J.F.; Rehfeldt, K.H.; Ashikhmina, E.; Bagameri, G.; Enriquez-Sarano, M.; Stulak, J.M.; Schaff, H.V.; Michelena, H.I. Adult Intraoperative Echocardiography: A Comprehensive Review of Current Practice. J. Am. Soc. Echocardiogr. 2020, 33, 735–755.e11.
- Wunderlich, N.; Siegel, R. Peri-interventional echo assessment for the MitraClip procedure. Eur. Heart J.-Cardiovasc. Imaging 2013, 14, 935–949.
