Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nutrition & Dietetics
Ageing is associated with changes in body composition, such as low muscle mass (sarcopenia), decreased grip strength or physical function (dynapenia), and accumulation of fat mass. When the accumulation of fat mass synergistically accompanies low muscle mass or reduced grip strength, it results in sarcopenic obesity and dynapenic obesity, respectively.
- sarcopenic obesity
- ageing
- dynapenic obesity 1. Introduction
1. Sarcopenic Obesity and Dynapenic Obesity
Obesity is a chronic disease [14] defined as the abnormal or excessive accumulation of fat [15]. It is expressed in various phenotypes, one of which is sarcopenic obesity [1,16]. The most widely used means of identifying obesity is to calculate the body mass index, taking the weight in kilograms, and dividing by the height in meters squared. Adult obesity is defined as a BMI of ≥30 Kg/m2 [5]. However, due to the endocrine and inflammatory role of adipose tissue, it is also necessary to classify obese conditions based on the distribution and composition of body fat. For this, some phenotypes of obesity have been described: normal weight obese, metabolically obese normal weight, metabolically healthy obese, metabolically unhealthy obese, and sarcopenic obese [1,17]. In older people, different phenotypes have been reported as: nonobese nondynapenic, overweight nondynapenic, obese nondynapenic, sarcopenic obese, overweight sarcopenic, nonobese dynapenic, and dynapenic obese [18,19,20] (Figure 1).
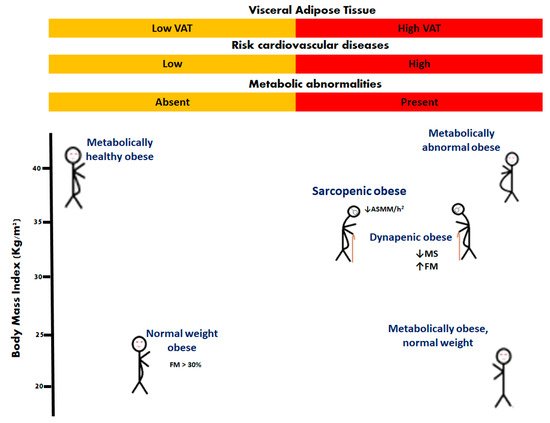
Figure 1. Phenotypes of obesity. In older people, sarcopenic obesity and dynapenic obesity are related to worsening disability. Appendicular skeletal muscle mass divided by height squared (ASMM/h2); fat mass (FM); muscle strength (MS).
It is well known that sarcopenia and obesity disorders are dependent on nutritional status [21], body composition, hormonal changes [22], strength and muscle mass [23], all of which act synergistically to increase the risk of disability [13].
Sarcopenic obesity (SO) is defined as obesity with the loss of muscle mass (ASMM/h2: < 5.18 Kg/m2 in women and <7.00 Kg/m2 in men), while dynapenic obesity (DO) is defined by the association of loss of leg muscle strength (<12 Kg women and <21 Kg men) or physical function; both phenotypes with an accumulation of body fat mass (>40% women and >28% men) or BMI ≥ 30 [24], particularly in those with comorbid diseases such as diabetes, arthritis, and cardiovascular and respiratory conditions [25,26,27]. It is important to mention that low muscle mass is associated with dynapenia and decreased motor capacity [28]. For the diagnosis of obesity in geriatric patients, the concept of DO is rarely considered; thus, patients with decreased muscle mass or low handgrip strength in sarcopenia may be included [29,30].
There are several mechanisms and factors related to the pathogenesis of SO and DO (Figure 2), such as (1) adipose tissue dysfunction characterized by adipocyte hyperplasia and hypertrophy [31,32]; (2) perilipins 5 is related to a decrease in the lipotoxicity and insulin resistance [33]; (3) systemic chronic sterile low-grade inflammation [34,35,36]; (4) vitamin D deficiency associated with handgrip strength but not with muscle mass [37]; (5) vitamin D receptor gene polymorphism of Fok1 associated with sarcopenia, lower gait speed, and lower handgrip strength [38]; (6) adipose tissue inflammation with an accumulation of macrophages and lymphocytes [39,40]; (7) during inflammation of adipose tissue, the accumulation of M1 macrophages around necrotic adipocytes produces the release of fatty acids. This is associated with the production of a greater amount of tumor necrosis factor-alpha (TNF-α), which releases more fatty acids from adipocytes, becoming a vicious circle that maintains the proinflammatory environment [41].
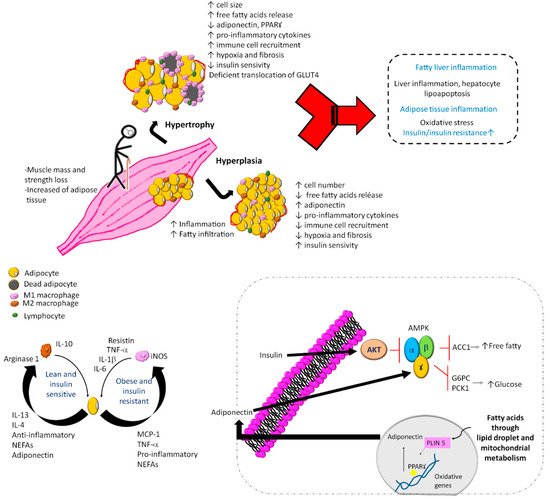
Figure 2. Mechanisms and factors related to the pathogenesis of sarcopenic obesity and dynapenic obesity. In sarcopenic obesity and dynapenic obesity, the muscle mass and strength loss with increased adipose tissue induces an inflammatory cascade and accumulation of immune cells, as well as leukocyte activation, adipogenesis, and adipocyte death. Added to physical inactivity, carbohydrate overload and lower protein intake cause a vicious circle of insulin resistance, where there is an increase in free fatty acids and M1 macrophages with alterations in mitochondrial metabolism by inactivation of regulators of energy homeostasis and inducers of regulated fatty acid oxidation, vitamin D deficiency, and D receptor gene polymorphism. G6PC, glucose-6-phosphatase; PKC1, phosphoenolpyruvate carboxykinase 1; AMPK, AMP-activated protein kinase; ACC1, acetyl-CoA carboxylase 1; NEFAs, nonesterified fatty acids; PLIN 5, perilipin 5; PPARꙋ, peroxisome proliferator-activated receptor gamma; GLUT4, glucose transporter type 4.
All these factors cause an asymptomatic inflammatory condition in hypertrophied adipose tissue with a high number of inflammatory cells, production of adipokines and other inflammatory cytokines [42]. The production of inflammatory cytokines motivates the arrival of immune cells, mainly macrophages, interferon gamma-producing TH1 lymphocytes (INF-γ), and CD8+ lymphocytes capable of initiating the inflammatory response [43,44].
In the skeletal muscle, fat droplets are accumulated as intermuscular adipose tissue and intramyocellular lipids (IMCLs) [45]. One characteristic of IMCLs is that they can induce a lipotoxic effect on muscles, which is characterized by impaired single-fiber contractility, leading to lower muscle strength and power in the elderly. This occurs because of the autophagy of muscle cells [46]. In the pathogenesis of sarcopenia, an important molecule identified at the neuromuscular junction is the C-terminal agrin fragment, which causes an age-dependent increase and muscle dysfunction [47]. Furthermore, during ageing, the change in muscle mass and weight gain (by lean mass) reflect the decrease in metabolic rate [48]. In addition, SO is related to elevated levels of IL-6, high-sensitivity C-reactive protein (hs-CRP) [49], IL-1 receptor antagonist, and soluble IL-6 receptor; all of these could contribute to apoptosis in myocytes and lead to a decrease in muscle mass and strength [50,51]. Thus, in SO, frailty and changes in immune function with age (immunosenescence) [52] are associated with physical inactivity and the reduction of energy expenditure, as well as impairment of movement and respiratory problems linked to metabolic alterations, leading to increased risk of comorbidity.
Vitamin D deficiency has also been found to be common in obese people [63]. This deficiency is associated with an increased risk of frailty, falls, and increased fracture risk in DO and SO [7,64,65]. Nevertheless, there are discrepancies in different disorder studies in which vitamin D is supplemented [36]. It should be remembered that vitamin D aids the body to absorb calcium, one of the main nutrients necessary for strong bones; vitamin D levels and vitamin D receptor gene polymorphism should be taken into consideration in SO and DO.
Although the affectations in SO and DO vary from one individual to another, there is a consensus on the higher risk of mortality in older adults in both cases [2]. Berens et al. evaluated the possible association between mortality and obesity in people, both with and without sarcopenia, and found that 75-year-old women with SO have a greater risk of dying at 10 years, compared to those without sarcopenia or obesity, while for 87-year-old obese men without sarcopenia, it was associated with a survival limit of up to four years. [66].
2. Sarcopenic Obesity, Dynapenic Obesity, and COVID-19
SO could increase the risk of severe complications and adverse outcomes in COVID-19 (Figure 3) [13,101]. Among the most notable findings of the COVID-19 patient are the effects of myalgia arthralgia, back pain, fatigue, and loss of grip strength [102], in addition to the fact that the SARS-CoV-2 infection mainly affects the epithelium of the lungs. Furthermore, other systems have also been involved, such as the immune [103], integumentary [104], neurological [105], digestive [106], genitourinary [107], cardiovascular [108], hematological [109], reproductive, and hormonal systems [110,111], causing very varied symptomatology. In the most severe cases, COVID-19 causes pneumonia, heart and kidney failure, and liver injury, thrombosis, shock, and even death. Twenty-five per cent of these patients develop severe lung disease that progresses to adult respiratory distress syndrome [112,113].
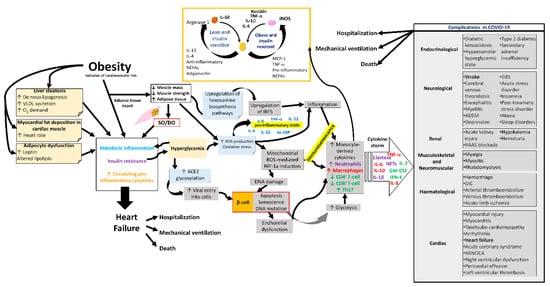
Figure 3. Complications in COVID-19 due to sarcopenic obesity and dynapenic obesity. Alterations in the metabolism, respiratory system, cardiovascular system, and immune system of the SO and/or DO patient with a propensity for complications during COVID-19. SO, sarcopenic obesity; DO, dynapenic obesity; ROS, reactive oxygen species; hs-CRP, high-sensitivity C-reactive protein; ACE2, angiotensin-converting enzyme 2; NETs, neutrophil extracellular traps; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN-γ, gamma interferon; HIF-1a, hypoxia-inducible factor-1α; IRF5, interferon regulatory factor 5; VLDL, very low-density lipoprotein; ADEM, acute disseminated encephalomyelitis; GBS, Guillain–Barré syndrome; RAAS, renin–angiotensin–aldosterone system; DIC, disseminated intravascular coagulation; MINOCA, myocardial infarction with nonobstructive coronaries.
Adipose tissue could function as a deposit for a wider viral spread with increased immune activation and cytokine amplification in patients associated with abnormal cytokine profiles [114]. SARS-CoV-2 infection depends on its binding to target cells facilitated by ACE2, which is expressed in various human tissue [115], particularly in the lungs, bowels, kidneys, and blood vessels [116].
SO patients manifest a higher prevalence of type 2 diabetes and hypertension than those without sarcopenic [117,118], associating them with a higher risk of respiratory disease and mortality [2]. In addition to this, a Chinese study found that people aged 65 years or older with type 2 diabetes are more susceptible to COVID-19 [119,120].
In COVID-19 patients, an association has been observed between decreased skeletal muscle area, low skeletal muscle radiodensity, and increased complications during their stay in the intensive care unit [121]. Likewise, low muscle quality and ectopic fat accumulation lead to invasive mechanical ventilation complications or even death, while increased muscle density is a protective factor [122].
COVID-19 affects immune cells and the expression of inflammatory molecules that increases with disease severity [123]. Lung infections by SARS-CoV-2 could lead to elevated blood sugar levels by adipose dysfunction in adiponectin and adiponectin/leptin ratios [124,125], making it difficult to control infections and metabolic diseases with sarcopenia or obesity [126]. Wilkinson et al. found that SO patients are approximately 2.6 times more likely to have severe COVID-19 infection than obese patients; however, sarcopenia alone did not increase the risk of severe COVID-19 [127].
Immunosenescence affects both the innate and adaptive immune response [128], leads to increased susceptibility to infections, reduces vaccination responses in frail elderly people, and increases the risk of chronic inflammatory diseases [52,129]. In COVID-19, not only do factors such as smoking, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, physical frailty, and C-reactive protein impact the severity/mortality of COVID-19, but also the components of DO, such as loss of grip strength and sarcopenia [130].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23158277
This entry is offline, you can click here to edit this entry!
