Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Bovine Respiratory Disease is considered one of the most common diseases within the Australian beef industry. During the transitioning period of young cattle going into the feedlot system they experience multiple stressors such as environmental changes, dehydration, and fatigue. These stressors negatively impact on the animals’ overall health by markedly increasing physiological stress and decreasing immune response making them more susceptible to diseases, such as bovine respiratory disease complex (BRD).
- algae
- fatty acids
- respiratory disease
- bovine
1. Bovine Respiratory Disease
The aetiology of BRD has still not been fully elucidated [1]. Current literature suggests a variety of factors, including stress, viral and/or bacterial agents, can synergistically contribute to overwhelm and dysregulate the animal’s immune system resulting in disease [2][3]. Figure 1 depicts a schematic overview of how BRD progresses within healthy cattle from initial infection; to the resulting pulmonary damage reported in BRD affected animals and the persistent infection that can occur in adult cattle. Figure 1 demonstrates that factors such stressors, viral agents and bacteria enter the animal and instigate necrosis or inhibition of the ciliated epithelial cells resulting in inability to remove pathogens from the upper respiratory tract. Consequently, BRD causing bacteria colonise and proliferate in the upper respiratory tract and into the lower respiratory tract. An immune response is triggered by the pattern recognition receptors (PRR) recognising the pathogen-associated molecular patterns (PAMP) which are produced by the BRD causing bacteria, this response includes the recruitment of neutrophils to the area as the first line of defence [3]. In conjunction, respiratory epithelial cells containing Toll-like receptors (TLR) and nucleotide oligomerisation domain (NOD)-like receptors are activated in the presence of PAMP, resulting in the production of pro-inflammatory cytokines [4]. The large production of pro-inflammatory cytokines and subsequent recruitment of neutrophils leads to the production of neutrophil extracellular traps (NET) which are capable of neutrophil-mediated pulmonary tissue damage by stimulating cell death of pulmonary epithelial cells [3].
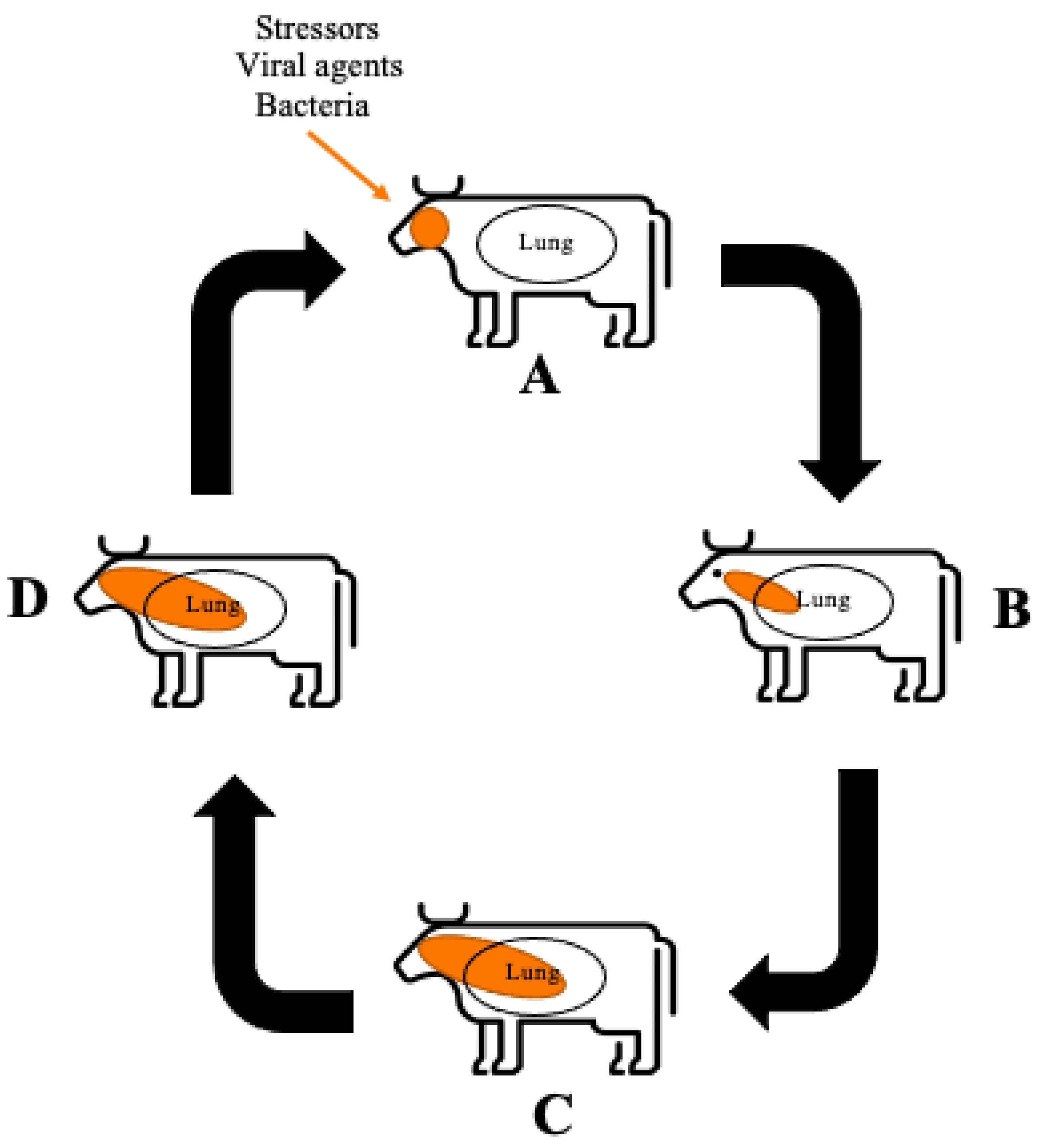
Figure 1. Schematic overview of BRD progression (A) The immune system is challenged allowing bacterial and viral infection to occur. Function of the ciliated epithelial cells is inhibited by necrosis or dysfunction. The immune system is activated via the PRR recognition of PAMPs. Neutrophils are recruited to the area as the first line of defence. (B) BRD causing bacteria proliferate in the upper respiratory area allowing infection of the bacteria into the lower respiratory area. The PRR recognise the PAMPs produced by the pathogens. Respiratory epithelial cells which contain TLR and NOD-like receptors also recognise PAMPs and in response produce pro-inflammatory cytokines. (C) Pro-inflammatory cytokines recruit a larger neutrophil response. Neutrophils undergo NETosis producing NETs stimulating cell death of surrounding pulmonary epithelial cells, resulting in pulmonary damage. (D) Persistent infection of BRD can occur through M. haemolytica producing a leukotoxin that binds to the [beta]2 integrin impairing the function of leukocytes, resulting in apoptosis, allowing the bacteria to evade host detection.
Ferraro et al. [5] identified that there was no clear definition of clinical symptoms associated with BRD, which made it difficult to diagnose from the signs exhibited by animals. Signs such as depressed mentation are often masked in cattle, due to their evolved survival mechanisms as prey animals, making the identification BRD difficult, resulting in low diagnosis rates [6]. Ferraro et al., (2021), later defined the clinical signs of BRD to include dyspnoea, tachypnoea, pyrexia, in addition to nasal discharge and coughing.
A consequence of BRD is pulmonary damage, due to inflammatory mediators contributing to the development of oedema and fibrous polyps within the lungs [7]. Angen et al. [8] showed that in 68% of the bronchoalveolar lavage fluid samples, collected from clinically healthy calves, tested positive for bacterium which are associated with the causation of BRD. With the natural presence of these bacteria in healthy cattle, any physiological stress to the animal has the potential to lower immune responses allowing the bacteria to overburden the animal. As a result, bacterial invasion has the potential to facilitate the pathogenesis of BRD due to alterations of the respiratory mucosa and immune system [4].
Stressors linked to BRD such as extreme temperatures, dust, injury, fatigue and dehydration are generally experienced when animals are transported to the feedlots, and also whilst at the feedlot [3]. Taylor et al. [9] reported sorting, loading and early transit were likely the most stressful components due to fasting, handling and potential injury resulting from handling and/or loading onto transportation. Human error has also been found to contribute to increased clinical implications such as dehydration in cattle due to lack of access to water when experiencing delayed transit or prolonged transportation. This is due to fatigued workers causing delays in processing the cattle into the feedlots and hence extending exposure to environmental stressors [9].
Viral agents predispose cattle to BRD in two distinct ways [3]. Firstly, the viral agent itself causes direct damage to respiratory clearance mechanisms by inhibiting function or activity of ciliated respiratory epithelial cells. Ciliated respiratory epithelial cells are responsible for trapping and expelling bacteria via the throat; preventing the bacteria from the deeper lung regions which remain relatively sterile in healthy cattle [4]. Viral agents inhibiting immunoprotective functions enables bacteria to proliferate to levels capable of causing disease [4][9]. Secondly, viral infections can interfere with the ability of the immune system to respond to bacterial infection. The viral infection interferes with the immune system through induction of apoptosis of specific T helper cells and their cell surface molecule CD4 [10].
Bacterial agents such as those listed in Table 1, have the capability to cause BRD through colonisation within the tonsil and mucous of the nasal passages due to ease of aerosol transmission during transport and in pens with animals in close proximity of others [4]. M. haemolytica is the major pathogen significantly associated with the pathogenesis of BRD, due to its high prevalence rate in clinically diagnosed cattle. It is suggested this is due the bacterium’s increasing antimicrobial resistance and the desirable conditions created when the animal is exposed to stressors allowing it to proliferate in the lung [11].
Table 1. Major bacterial and viral species associated with BRD.
| Bacteria Species | Viral Species | Reference |
|---|---|---|
| Histophilus somni | Bovine herpesvirus (BHV-1) | [3] |
| Mannheimia haemolytica | Bovine parainfluenza virus 3 (PIV) | [3] |
| Pasteurella multocida | Bovine respiratory syncytial virus (BRSV) | [3] |
| Mycoplasma bovis | Bovine viral diarrhoea virus (BVDV) | [3] |
| Arcanobacterium pyogenes | [8] |
Airway and Lung Epithelia
The respiratory tract of cattle differs to other animals due to its relatively long tracheobronchial tree and associated increased dead space within the lung [4]. This dead space means that the gas transit time within the lung and the surface area is increased allowing for particulate matter to deposit creating opportunity for bacteria to proliferate in ideal growing conditions of a relatively warm and moist area.
The respiratory tract is considered the first line of defence of the animals’ innate immune system as it is where the bacteria initially come into contact with the animal. Bacteria are inhaled as droplets via aerosol transmission into the nasal passages where they can adhere and colonise the epithelial surface [12]. The epithelial cilia of the trachea are responsible for the removal of pathogens; however, viral agents such as BRSV and PIV can cause ciliary dysfunction and necrosis delaying the removal of bacteria from the airway [3]. Upper respiratory tract damage is also caused by BHV-1 by infecting the epithelium layer subsequently causing rhinotracheitis. The lesions caused effectively destroy the normal processes of pathogen removal via inhibiting cilia movement and, allowing commensal bacteria such as M. haemolytica to colonise and proliferate in the lower respiratory tract causing disease [10]. Furthermore, dehydration has also been shown to disrupt the air-surface liquid lining (ASL) within the upper respiratory tract by increasing the viscosity of the lining. As a result, this decreases the animal’s ability to trap pathogens entering the respiratory system and to eliminate particulates via coughing or sneezing [4].
2. Immune Responses
The immune system is responsible for recognising the PAMPs produced by the BRD causing pathogens. The response involves the production of pro-inflammatory cytokines from infected pulmonary epithelial cells in conjunction with neutrophils to the site of infection to mount the appropriate antibody response. It is also this immune response that contributes to the pulmonary tissue damage reported in infected cattle [4][13]. However, BRD causing bacteria M. haemolytica is able to evade host detection by producing a cytolytic exotoxin resulting in persistent infection within adult cattle [10].
2.1. Pro-Inflammatory Cytokines
With the immune system activated, neutrophils, and monocytes are recruited to the site of infection in response to pro-inflammatory cytokines [3], such as interleukin-1 (IL-1), interleukin-8 (IL-8), and tumour necrosis factor-α (TNFα) [4][13]. With the recruitment of these cytokines there is also the attraction of dendritic cells, NK, T, and B cells as part of the antibody response to the infection [3]. In particular, the chemokine receptor CXCR1 gene as identified by Lindholm-Perry et al. [14], contributes to the attraction and activation of neutrophils at the site of inflammation when stimulated by IL-8. Pro-inflammatory cytokines, whilst being the main promoters for effector element development within the immune response [10], also have an important role in the physiological signs exhibited by the animal as they are capable of crossing the blood–brain barrier allowing the modification to the animals thermoregulation causing fever, as well as the ability to modify behaviour creating a desire to sleep [15]. These are the typical clinical signs as observed by cattle with BRD. It has been suggested that the pulmonary damage seen in BRD affected cattle is due to the rapid secretion of pro-inflammatory cytokines triggering a large recruitment of neutrophils to the site of infection. Coinfection of BRD causing bacteria with viral pathogens has also been shown to intensify this cytokine production [3].
M. haemolytica produces an exotoxin that is cytolytic to white blood cells and has hence been termed as a leukotoxin [10]. During the bacteria’s growth phase, leukotoxin production is at its peak, causing leukocytes to release eicosanoids and cytokines. Prolonged periods of high leukotoxin levels impair the function of the leukocytes resulting in apoptosis [16]. The leukotoxin is able to cause the destruction of leukocytes due to its ability to bind to the leukocyte specific integrin [beta]2. The [beta]2 integrin is a transmembrane protein that allows the leukocyte to perform functions of phagocytosis, antigen presentation and focusing in on areas of inflammation [10]. M. haemolytica binding properties facilitates evading host detection and reduces the production of pro-inflammatory cytokines and may partially explain the persistent infection seen in adult cattle [17].
Ozkanlar, Aktas, Kaynar, Ozkanlar, Kirecci and Yildiz [17] describes cytokine levels as significantly elevated after initial infection with a BRD causing bacterial agent with levels decreasing once the calves began exhibiting clinical signs such as fever and tachypnoea. This is also supported by studies performed by Burciaga-Robles et al. [18] reporting an increase in cytokine levels within cattle exposed to M. haemolytica and also recorded an increase in Interleukin-1β (IL-1β) in persistently infected cattle. Both authors recognise that exposure to BRD causing agents result in an increase in pro-inflammatory cytokines which contributes to extensive pulmonary tissue damage. However, further investigation is required to evaluate the long-term effects of BRD in cattle.
2.2. Neutrophils
The neutrophil is one of the first cell types to be recruited to the site of infection due to its phagocytic role of consuming foreign substances. Whilst they have an important role in the removal of extracellular bacterial infections, neutrophils are also considered a contributor to the pulmonary tissue damage caused by BRD infection as they contribute to the formation of intralobular oedema, alveolar necrosis and occlusion of the small airway [3][19][20]. This damage is due to neutrophils releasing NETs, which contain extracellular deoxyribonucleic acid (DNA) and proteins that stimulate a specific cell death process known as NETosis [3][21]. NETs have been reported to contribute to neutrophil-mediated pulmonary tissue damage by stimulating cell death of pulmonary epithelial cells. Breider, Walker, Hopkins, Schultz and Bowersock [19] reported calves with induced neutropenia, using hydroxyurea, had a markedly decreased number of alveolar lesions compared to calves with no neutrophil alterations. Radi et al. [22] supports these results with a similar study, demonstrating a decrease in pulmonary lesions within calves that were subjected to induced neutropenia. This supports the concept that neutrophils do play an active role in the pathogenesis of BRD by exacerbating pulmonary tissue damage in the presence of pathogens.
2.3. Pattern Recognition Receptors
Located within the innate immune system are PRRs which recognise PAMPs [3]. All pathogens within the BRD complex produce some type of PAMP that activates the innate immune system within the animal. In acute inflammation, neutrophils also recognise PAMPs, which stimulates other immune cells including natural killer (NK) T cells, T cells, B cells, and plasmacytoid dendritic cells to interact with the PAMP through activation of PRR. Furthermore, respiratory epithelial cells containing Toll-like receptors (TLR) and nucleotide oligomerisation domain (NOD)-like receptors are activated in the presence of PAMP, resulting in the production of pro-inflammatory cytokines [4]. The immune systems recognition of PAMPs within the body allows for the necessary immune response to BRD. However, the recruitment of pro-inflammatory cytokines and neutrophils as a result of PAMP recognition within the immune system is also a contributing factor to the large immune response to BRD bacteria that is capable of causing pulmonary tissue damage as previously described.
This entry is adapted from the peer-reviewed paper 10.3390/ani12151943
References
- Smith, R.A.; Step, D.L.; Woolums, A.R. Bovine respiratory disease: Looking back and looking forward, what do we see? Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 239–251.
- Hay, K.E.; Morton, J.M.; Clements, A.C.; Mahony, T.J.; Barnes, T.S. Associations between feedlot management practices and bovine respiratory disease in Australian feedlot cattle. Prev. Vet. Med. 2016, 128, 23–32.
- McGill, J.L.; Sacco, R.E. The immunology of bovine respiratory disease: Recent advancements. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 333–348.
- Ackermann, M.R.; Derscheid, R.; Roth, J.A. Innate immunology of bovine respiratory disease. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 215–228.
- Ferraro, S.; Fecteau, G.; Dubuc, J.; Francoz, D.; Rousseau, M.; Roy, J.P.; Buczinski, S. Scoping review on clinical definition of bovine respiratory disease complex and related clinical signs in dairy cows. J. Dairy Sci. 2021, 7095–7108.
- Timsit, E.; Dendukuri, N.; Schiller, I.; Buczinski, S. Diagnostic accuracy of clinical illness for bovine respiratory disease (BRD) diagnosis in beef cattle placed in feedlots: A systematic literature review and hierarchical Bayesian latent-class meta-analysis. Prev. Vet. Med. 2016, 135, 67–73.
- Bassel, L.L.; Tabatabaei, S.; Caswell, J.L. Host tolerance to infection with the bacteria that cause bovine respiratory disease. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 349–359.
- Angen, O.; Thomsen, J.; Larsen, L.E.; Larsen, J.; Kokotovic, B.; Heegaard, P.M.; Enemark, J.M. Respiratory disease in calves: Microbiological investigations on trans-tracheally aspirated bronchoalveolar fluid and acute phase protein response. Vet. Microbiol. 2009, 137, 165–171.
- Taylor, J.D.; Fulton, R.W.; Lehenbauer, T.W.; Step, D.L.; Confer, A.W. The epidemiology of bovine respiratory disease: What is the eveidence for predisposing factors? Can. Vet. J. 2010, 51, 1095–1102.
- Srikumaran, S.; Kelling, C.L.; Ambagala, A. Immune evasion by pathogens of bovine respiratory disease complex. Anim. Health Res. Rev. 2007, 8, 215–229.
- Zecchinon, L.; Fett, T.; Desmecht, D. How Mannheimia haemolytica defeats host defence through a kiss of death mechanism. Vet. Res. 2005, 36, 133–156.
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 381–394.
- Ellis, J.A. The immunology of the bovine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 535–550.
- Lindholm-Perry, A.K.; Kuehn, L.A.; McDaneld, T.G.; Miles, J.R.; Workman, A.M.; Chitko-McKown, C.G.; Keele, J.W. Complete blood count data and leukocyte expression of cytokine genes and cytokine receptor genes associated with bovine respiratory disease in calves. BMC Res. Notes 2018, 11, 786.
- Gershwin, L.J. Immunology of bovine respiratory syncytial virus infection of cattle. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 253–257.
- Czuprynski, C.J.; Charles, J.; Leite, F.; Sylte, M.; Knuckleburg, C.; Schultz, R.; Inzana, T.; Behling-Kelly, E.; Corbeil, L. Complexities of the pathogenesis of Mannheimia haemolytica and Haemophilus somnus infections: Challenges and potential opportunities for prevention? Anim. Health Res. Rev. 2004, 5, 277–282.
- Ozkanlar, Y.; Aktas, M.S.; Kaynar, O.; Ozkanlar, S.; Kirecci, E.; Yildiz, L. Bovine respiratory disease in naturally infected calves: Clinical signs, blood gases and cytokine response. Vet. Med. J. 2012, 163, 123–130.
- Burciaga-Robles, L.O.; Step, D.L.; Krehbiel, C.R.; Holland, B.P.; Richards, C.J.; Montelongo, M.A.; Confer, A.W.; Fulton, R.W. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and subsequent infection with Mannheima haemolytica on clinical signs and immune variables: Model for bovine respiratory disease via viral and bacterial interaction. J. Anim. Sci. 2010, 88, 2179–2188.
- Breider, M.A.; Walker, R.D.; Hopkins, F.M.; Schultz, T.W.; Bowersock, T.L. Pulmonary lesions induced by Pasteurella haemolytica in neutrophil sufficient and neutrophil deficient calves. Can. J. Vet. Res. 1988, 52, 205–209.
- Bassel, L.L.; Caswell, J.L. Bovine neutrophils in health and disease. Cell Tissue Res. 2018, 371, 617–637.
- Aulik, N.A.; Hellenbrand, K.M.; Klos, H.; Czuprynski, C.J. Mannheimia haemolytica and its leukotoxin cause neutrophil extracellular trap formation by bovine neutrophils. Infect. Immun. 2010, 78, 4454–4466.
- Radi, Z.A.; Caverly, J.M.; Dixon, R.A.; Brogden, K.A.; Ackermann, M.R. Effects of the synthetic selectin inhibitor TBC1269 on tissue damage during acute Mannheimia haemolytica-induced pneumonia in neonatal calves. Am. J. Vet. Res. 2001, 62, 17–22.
This entry is offline, you can click here to edit this entry!
