Salicylic acid (SA) is a very simple phenolic compound (a C7H6O3 compound composed of an aromatic ring, one carboxylic and a hydroxyl group) and this simplicity contrasts with its high versatility and the involvement of SA in several plant processes either in optimal conditions or in plants facing environmental cues, including heavy metal (HM) stress.
1. Introduction
Salicylic acid (SA) (from Latin
Salix, willow tree), also known as
ortho-hydroxybenzoic acid, is a phenolic derivative widely distributed in the plant kingdom and is known as a regulator of several physiological and biochemical processes such as thermogenesis, plant signaling or plant defense, and response to biotic and abiotic stress [
1,
2].
From a chemical point of view, SA belongs to a large group of plant phenolics, and SA can be isolated in plants in both free and conjugated form. In particular, the conjugated form proceeds from the methylation, hydroxylation, and/or glucosylation of the aromatic ring [
3,
4].
Salicin, one of the natural SA derivatives, was first isolated from the bark of the willow tree (
Salix sp.) by Johan Büchner in 1828 [
5,
6]. Successively, it was discovered that almost all the willow trees including
Salix alba,
S. purpurea, S. fragilis, and
S. daphnoides were particularly rich in this natural compound, in which the concentration in plants significantly fluctuates during the different seasons (highest content during spring and summer, lowest content during autumn and winter [
7]) reaching values of 3 mg/g of fresh biomass in plants of
S. laponum [
8]. The first scientist who was able to identify this natural compound in species different from Salix sp. was the Italian chemist Raffaele Piria in the late 1838, who obtained SA in both flower and buds of the European species
Spiraea ulmaria successively renamed as
Filipendula ulmaria (L.) Maxim. The discovery that this molecule was not exclusive to the
Salix genus has opened the door to the study of its biosynthesis, as well as its biochemical and physiological role in plants and in 1899 the Bayer Company formulated a new drug known today as aspirin [
9].
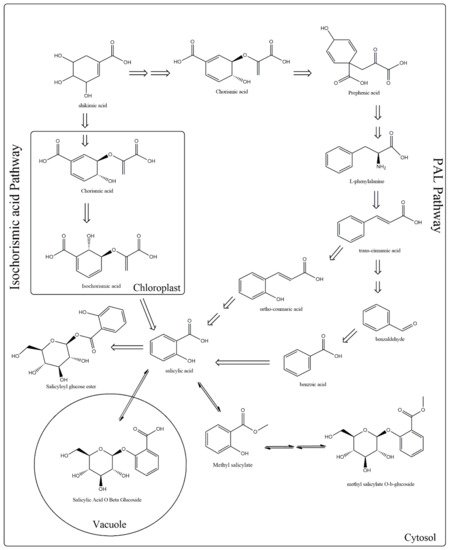
Concerning the biosynthesis of SA, it is known to be produced through the shikimate pathway by two metabolic routes (
Figure 1). In the first discovered route, also known as phenylalanine route, occuring in the cytoplasm of the cell, the enzyme phenylalanine ammonia lyase (PAL) converts phenylalanine (Phe) to
trans-cinnamic acid (t-CA), which gets oxidized to benzoic acid (BA). Subsequently, the enzyme benzoic-acid-2-hydroxylase (BA2H) catalyzes the hydroxylation of BA aromatic ring and leads to SA formation. The enzymatic conversion of BA into SA by BA2H requires the presence of hydrogen peroxide (H
2O
2) [
10,
11,
12].
Figure 1. Metabolic pathways involved in the biosynthesis of salicylic acid (SA). Plants use two pathways for SA production, the phenylalanine ammonia-lyase (PAL) (which is divided into two sub-pathways, benzoic acid, and o-coumaric acid) and the isochorismate. In both routes, shikimate serves as a precursor.
The first evidences for the first route were given by Ellis and Amrchein [
13], who observed that feeding
Gaultheria procumbens plants with labeled 14C-benzoic acid or 14C-cinnamic acid resulted in the production of labeled SA. Successively, Yalpani et al. [
14] and Silverman et al. [
15], working on rice and tobacco, proposed that the side chain of
trans-cinnamic acid is decarboxylated to generate BA. Then, BA is hydroxylated at the C2 position forming SA. Anyway, recent results indicated that benzoyl glucose, a conjugated form of BA, is more likely to be the direct precursor of SA [
12,
14].
The second route is called isochorismate (IC) pathway and occurs in the chloroplast [
16,
17,
18]. In plants, chorismate is transformed to isochorismate and then to SA, a reaction which is catalyzed by two enzymes isochorismate synthase (ICS) and isochorismate pyruvate lyase (IPL). Recent studies carried on
Arabidopsis thaliana demonstrated that the ~90% of defense-related SA is produced from isochorismate generated by the plastid-localized isochorismate synthase1, whereas ~10% is derived from the cytosolic PAL pathway [
1,
17].
From the physiological point of view, it is known that SA plays a pivotal role in the regulation of plant growth, development, in defense from biotic and abiotic stress, and in plant immune responses [
4,
19,
20,
21,
22,
23].
For several years, SA was believed to be just one of the several phenolic compounds synthetized by plants with relatively low importance [
5,
16]. In 1974, after more than a hundred of years from its discovery, it was provided the first evidence that SA could play a role as plant hormone, when Clealand and Ajami [
24] observed that SA was a mobile signaling molecule localized in the phloem inducing flowering in different plant species.
However, the final evidence that SA was a plant hormone was only provided several years later by Raskin et al. [
25], who described its role during the thermogenesis in
Sauromatum guttatum.
From that moment, an exponential increase of manuscripts focused on SA (acting alone or in concert with other plant hormones) as a plant growth regulator, signaling molecule, as well as plant elicitor protecting plants from biotic and abiotic stress, was observed [
22,
23,
26,
27,
28,
29,
30,
31].
Recently, it has also been demonstrated that SA could play a pivotal role in protecting plants from environmental stress, including heavy metals (HM). In fact, several recent manuscripts reported that SA can alleviate HM toxicity influencing both their uptake and/or accumulation in plant organs [
32,
33,
34,
35,
36,
37,
38], as well as scavenging of reactive oxygen species (ROS) and/or decreasing their accumulation and/or enhancing the antioxidant defense system [
39,
40,
41,
42], protecting membrane stability and integrity [
43], interacting with plant hormones [
44], upregulating heme oxygenase [
45], and improving the performance of the photosynthetic machinery [
42,
46,
47].
2. HM Stress and Its Impacts on Plants
Metals and metalloids with atomic density more than 6 g cm
−3 are defined as (HM). Both, essential elements, micronutrients that are required in low concentration (e.g., Cu, Cr, Co, and Zn), and nonessential metals such as Pb, Cd, Hg, are incorporated in this group [
48,
49]. Increased concentration of both essential and nonessential elements is phytotoxic to flora and fauna [
50,
51]. Heavy metal contamination has become a serious environmental problem worldwide. The increased industrialization, injudicious population growth, and urbanization releases HM that compromise soil and water and pose harms to living biota due to their biomagnification through the food chain [
52]. Natural activities such as eruption of volcano and erosion of rocks have contribute in increasing the release of toxic elements to the environment; however, increased human activities such as mining, painting, and refining have enhanced their concentration in the biosphere [
53,
54,
55].
Soil pollution by HM poses serious concerns to the biotic and abiotic components of the ecosystem [
56]. The increased amount of HM in soil leads to greater uptake by plants that can reduce plant growth, biomass, photosynthesis, crop yield, and quality in plant [
57]. From a biological point of view, the top soil is the most active zone of soil that accumulates a large amount of toxic metals that poses serious concern to the environment [
49,
58,
59].
The increased level of HM accumulation in plant organs negatively affects the cell metabolism in plants [
60]. The different physiological activities in plants such as protein metabolism, photosynthesis, respiration, and morphogenesis are naturally affected by a high concentration of toxic compounds, such as HM [
53,
54,
61,
62]. For instance, Rascio et al. [
63] documented a decreased root growth and altered morphogenesis in rice seedlings upon treatment with Cd. Many plant species such as
Brassica napus,
Helianthus annuus,
Thalaspi caerulescens,
Vigna radiata showed inhibition in photosynthesis in response to Cd treatment [
64,
65,
66,
67,
68]. Recently, Tandon and Srivastava [
69] investigated the Pb effect on the morphology and metabolism of
Sesamum indicum and found that the increasing concentration of metal affected the growth of the plant. Further, the plant showed severe symptoms of chlorosis, necrosis and reduced chlorophyll, and protein content at higher doses of Pb [
69].
The major outcome of metal toxicity is the peaked production of ROS due to impairment of photosynthetic process by HM [
70]. ROS such as hydroxyl, superoxide, and hydrogen peroxide are produced as by-product during electron transport in photosynthesis and respiration pathways [
71]. Under physiological conditions, ROS play a multitude of signaling roles in plants, as well as in other organisms and they take part in a finely-tuned and well-orchestrated regulatory network [
72,
73]. ROS are indeed integrated into a complex regulatory system in plants which encompasses ROS, plant hormones (e.g., ethylene (ET) and abscisic acid (ABA)), signaling molecules (e.g., salicylic acid (SA) and jasmonic acid (JA)), and secondary messengers (e.g., Ca
2+) [
74,
75]. However, when ROS production exceeds the physiological levels, their accumulation can lead to oxidative stress in the cells, that cause lipids peroxidation, macromolecular degradation, membrane disruption, DNA breakage, and ion leakage in plants [
70,
74,
75]. For instance, Kaur et al. [
76] explored Pb-induced ultrastructural changes in roots of wheat and concluded that Pb inhibited root growth, caused ROS generation, and disrupted mitochondrial and nuclear integrity in the tested plant.
The enhanced generation of ROS in the plant cell is controlled by a complex network of antioxidant machinery that maintains ROS homeostasis in the cell [
77]. Plants have a finely-tuned and well-orchestrated defense system that includes enzymatic antioxidants such as catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione peroxidase (GPX) and glutathione reductase (GR), and nonenzymatic antioxidants such as ascorbic acid, glutathione, alkaloids, phenol compounds, and α-tocopherol for scavenging excessive ROS [
49,
61]. Moreover, phytohormones such as auxins, gibberellins, cytokinins, abscisic acid, ethylene, brassinosteroids, jasmonic acid, and SA take part in the defensive mechanism of plants against HM stress.
3. Physiological Roles of SA in Plants Under HM Stress
Concerning the physiological role in plants, SA is known to play a pivotal role in regulating plant morphology, development, flowering, and stomatal closure [
78,
79]. SA also affects seedling germination, cell growth, and nodulation in legumes [
80]. Khan et al. [
81] reported increased leaf area and dry weight production in corn and soybean in response to SA. Furthermore, Hussein et al. [
82] reported pot studies that documented improved growth, leaf number, dry biomass, and stem diameter in wheat plants when leaves were sprayed with SA. The rate of transpiration and stomatal index of plants increased in response to supplementation of SA [
81]. The pigment concentration in wheat seeds significantly enhanced upon exposure to a low concentration (10
−5 M) of SA. However, foliar application of SA reduced transpiration rate in test plants,
Phaseolus vulgaris and
Commelina communis which might be due to the SA-evoked stomatal closure [
83,
84,
85,
86,
87]. Moreover, SA has been reported to increase the shelf life of cut flowers of rose and defer senescence by controlling water level in rose plants [
86].
Plant growth regulators or phytohormones especially, gibberellins, auxin, cytokinins, ethylene, brassinosteroids, and also SA play a key role in providing HM tolerance in plants [
83]. SA, a phenolic plant hormone, regulates photosynthesis, respiration, and antioxidant defense mechanism in plants under different abiotic stress such as high temperature, salinity, and HM [
78,
88,
89]. SA pretreatment provides protection from various metals such as Pb, Hg, Cd, in different plants [
90,
91,
92].
Supplementation of SA in combination with plant growth promoting bacteria reduces Cr-induced oxidative damage in maize by enhancing activities of antioxidant and nonantioxidant enzymes [
93,
94]. Earlier, Song et al. [
95] reported SA mediated enhancement in the activities of CAT and SOD enzymes in barley leaves under Zn, Cu, and Mn stress. Further, carbohydrate metabolism in Cr-treated maize plants improved upon exposure to SA [
94]. Alleviation of Cd toxicity was reported in mustard plants in response to exogenous treatment of SA [
93]. Recently, SA treatment mitigated Cd stress in
Brassica juncea plants and enhanced growth and photosynthesis in plants. Moreover, supplementation of SA reduced reactive oxygen species levels by strengthening the antioxidant defense system in plants and provides stability to the plant membrane [
96]. The exogenous application of SA upregulates the antioxidant system, improves growth and yield, and results in lowering of oxidative damage under Pb stress in
B. campestris [
97].
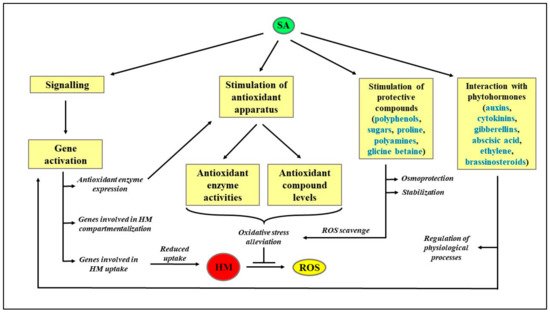
A schematization of the protective role exerted by SA in HM-stressed plants is reported in Figure 2, whereas a literature survey on the effect of different HM on plant metabolism is reported in Table 1.
Figure 2. Schematization of the protective role exerted by SA in HM-stressed plants. HM: Heavy metals; ROS: Reactive oxygen species; SA: Salicylic acid.
Table 1. Salicylic acid (SA) effect on different heavy metals (HM) stressed plants.
|
HM
|
Species
|
Effects of SA in plant metabolism
|
References
|
|
Cd
|
Lemna minor L.
|
Induced a reduction of Cd uptake, the maintenance of ionic homeostasis, improvement of PAL activity, activation of ROS scavenger and of the heat shock proteins.
|
[98]
|
|
Oryza sativa L.
|
SA in association with NO reduced Cd uptake and accumulation, as well as ROS accumulation and malondialdehyde production through the maintenance of ascorbate and glutathione levels, and redox status. Improved the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase, and mono dehydroascorbate reductase.
|
[41]
|
|
Brassica juncea L. Czern
|
Stimulating the stomatal activity and pore size, alleviated the inhibitory effect of Cd on photosynthesis. The Cd-generated oxidative burst was reduced via enhanced antioxidant activity (CAT and SOD) promoted by SA.
|
[96]
|
|
Nymphaea tetragona
|
SA pretreatment decreased Cd concentration and increased the contents of glutathione, nonprotein thiol and phytochelatins.
|
[99]
|
|
Solanum tuberosum
|
Cd stress increased endogenous SA level, relative water content, chlorophyll, and proline. Reduced lipid peroxidation, H2O2 and O2-. SA stimulated enzymatic antioxidants.
|
[100]
|
|
Triticum aestivum L.
|
Induced a transient upregulation of protein kinases (SIPK).
|
[101]
|
|
Mentha piperita
|
Improved photosynthesis by enhancing activity of RuBisCo and carbonic anhydrase. Reduced the oxidative stress by mitigating the production of free radicals by the maintenance of reduced glutathione pool and free radical scavenging enzymes. Furthermore, restored essential oils production previously affected by Cd.
|
[102]
|
|
Pb
|
Brassica juncea L. Czern
|
Co-application of 24-epibrassinolide and SA mitigates the negative effects of Pb, by lowering Pb metal uptake and enhancing the heavy metal tolerance index, antioxidative capacities, organic acid levels, phenolic content, water content, and relative water content.
|
[37]
|
|
Zea mays L.
|
Improved nitrate reductase activity, glutathione content, and regulated the amino acids metabolism.
|
[103]
|
|
Triticum aestivum L.
|
Suppressed chlorophyll degradation, electrolyte leakage, and malondialdehyde accumulation. Furthermore, enhanced the production of total soluble carbohydrates, proline, and the activities of SOD, CAT, and peroxidases.
|
[104]
|
|
Brassica campestris L.
|
Improved plant growth and yield upregulating, in the antioxidant defense system, both enzymatic and nonenzymatic components.
|
[93]
|
|
Zea mays L.
|
In combination with sodium hydrosulfide reduced arginine, proline, and methionine accumulation and increased nitric oxide and glycine betaine content. Moreover, it regulated the expression of ZmSAMD and ZmACS6 genes (genes involved in methionine metabolism).
|
[105]
|
|
As
|
Trigonella foenum-graecum L.
|
Enhanced root growth and increased protein content, free amino acids, and soluble sugars in both cotyledons and radicles. Moreover, it enhanced the activity of hydrolytic enzymes (α- and β-amylase).
|
[106]
|
|
Artemisia annua L.
|
Increased endogenous SA level, reduced H2O2 and O2− generation, as well as lipid peroxidation. Reverted biomass and chlorophyll content. Increased artemisinin, and dihydroartemisinic acid level. Upregulated the expression of four key artemisinin biosynthetic pathway genes (CYP71AV1, ALDH1, ADS, and DBR2).
|
[107]
|
|
Artemisia annua L.
|
Upregulated proteins related to energy metabolism, photosynthesis, secondary metabolism, transcriptional regulators, transport proteins, and proteins related to lipid metabolism.
|
[108]
|
|
Helianthus annuus L.
|
Alleviated the negative effect of As on growth and decreased oxidative injuries through the increasing of the enzymatic activity of ROS scavengers such as CAT, ascorbate peroxidase (APX), and glutathione peroxidase, whereas the activity of SOD and guaiacol peroxidase activities was reduced.
|
[109]
|
|
Oryza sativa L.
|
As enhanced endogenous level of SA and NO level through the enhancement of nitrate reductase activity.
|
[110]
|
|
Cr
|
Sorghum bicolor L.
|
Increased both APX and hydrogen peroxide content and decreased the peroxidase activity and ascorbic acid content.
|
[111]
|
|
Brassica napus L.
|
Increased dry biomass, enhanced plant growth, and strengthened the reactive oxygen scavenging system by improving the activity in Cr-damaged organelles.
|
[112]
|
|
Oryza sativa L.
|
Reduced the concentration and translocation of Cr in shoots but not in roots, suggesting a detoxification strategy based on Cr sequestration in roots. Increased growth parameters, membrane stability, and protein content.
|
[113]
|
|
Ni
|
Brassica juncea L. Czern. & Coss.
|
Restored growth and photosynthesis increasing the activities of enzymes associated with antioxidant systems, especially the glyoxalase system and the ascorbate–glutathione cycle (AsA–GSH) cycle. It had an additive effect on the activities of the ascorbate and glutathione pools, and the AsA–GSH enzymes and restored the content of mineral nutrient.
|
[114]
|
|
Eleusine coracana L.
|
Inhibited Ni transport from roots to shoots, increased chlorophyll content, and the photosynthetic rate, increased the uptake of mineral content, reduced H2O2 and proline content, and enhanced the activity of antioxidant enzymes (SOD, CAT, APX).
|
[115]
|
|
Melissa officinalis L.
|
Decreased Ni transport to the shoots, increased carotenoid content, induced a significant decrease in electrolyte leakage in stressed plants.
|
[116]
|
|
Alyssum inflatum Náyr.
|
Mitigated Ni oxidative effects by reducing H2O2 concentration. Reversed the detrimental effects of Ni on carotenoid content and reduced the proline content.
|
[117]
|
|
Co
|
Triticum aestivum L.
|
Decreased the accumulation of H2O2 and MDA and improved the activity of antioxidant enzymes.
|
[40]
|
|
Cu
|
Gossypium barbadense L.
|
Limited Cu translocation and improved the activities of antioxidant enzymes.
|
[118]
|
|
Zea mays L.
|
Lowered Cu and H2O2 accumulation in roots. Induced a reduction of MnSODII activity accompanied by a decrease in H2O2 concentration.
|
[119]
|
|
Zea mays L.
|
Increased the biomass, root and shoot length, number and leaves area.
|
[119]
|
This entry is adapted from the peer-reviewed paper 10.3390/molecules25030540