The term ‘ferroptosis’, first proposed in 2012, refers to a programmed cell death resulting from iron-dependent lipid peroxidation accumulation. Ferroptosis is distinct from other previously established regulated cell deaths and has specific morphological, biochemical, and genetic characteristics. The regulation of ferroptosis is associated with multiple signal pathways, and there is increasing evidence to suggest its participation in the regulation of numerous diseases. In this paper, we describe the basic features and the regulation mechanisms of ferroptosis, and analyze the application of ferroptosis in chronic diseases such as cancer, nervous system diseases, metabolic diseases, and inflammatory bowel diseases. We also describe the regulatory effects of food-borne active ingredients on ferroptosis.
2. Basic Characteristics of Ferroptosis
2.1. Morphological Features
Ferroptosis has morphological, biochemical, and genetic features that are unique in comparison with apoptosis, autophagy, necroptosis, and pyroptosis (
Table 1) [
1,
2,
3]. Cells that undergo ferroptosis generally show a necrosis-like morphological transformation, including cell membrane rupture, cytoplasmic swelling, and moderate chromatin condensation [
4]. At the ultrastructural level, ferroptotic cells usually present mitochondrial shrinkage, an increase in membrane density, reduced or absent cristae, and rupturing of the outer membrane [
2]. Since autophagy promotes ferroptosis, autophagy-related ultrastructures, such as double-membrane autophagosomes and various lysosome-related vesicles, are often seen in ferroptotic cells or tissue [
5].
Table 1. Characteristics of ferroptosis, apoptosis, autophagy, necroptosis and pyroptosis.
2.2. Biochemical Features
The two main biochemical features of ferroptosis are iron accumulation and lipid peroxidation. Intracellular free ferrous iron accumulation generates excessive reactive oxygen species (ROS) directly via the Fenton reaction, thereby triggering lipid peroxidation and ferroptosis. Erastin induces ferroptosis by decreasing ferroportin (FPN) and increasing the level of ferrous ions (Fe
2+) in endometriosis [
6]. ROS-mediated autophagy results in ferroptosis via the degradation of ferritin and inducement of transferrin receptor (TFR1) expression to increase intracellular iron levels [
7]. Iron overload in the motor cortex has been reported to trigger the ferroptosis of neuronal cells after spinal cord injury [
8]. Ferroptosis inhibition attenuates atherosclerosis via the alleviation of lipid peroxidation [
9].
2.3. Genetic Features
Some genes are overexpressed in ferroptosis. For example, prostaglandin-endoperoxide synthase 2 (PTGS2/COX2) is known to play an important role in prostaglandin biosynthesis [
10], while Acyl-CoA synthetase long-chain family member 4 (ACSL4) is an enzyme that increases the polyunsaturated fatty acid (PUFA) content in phospholipids, making them more sensitive to ferroptosis [
11]. Other genes, such as glutathione peroxidase 4 (GPX4) and ferritin heavy chain 1 (FTH1), are downregulated in ferroptosis. GPX4 converts the cytotoxic lipid peroxides (L-OOH) into the corresponding alcohols (L-OH) under glutathione (GSH), and the suppression of GPX4 activity leads to lipid peroxides accumulation and ferroptosis. FTH1 is an iron-storage protein and, therefore, the reduction of its expression facilitates iron accumulation and ferroptosis.
3. Regulation of Ferroptosis
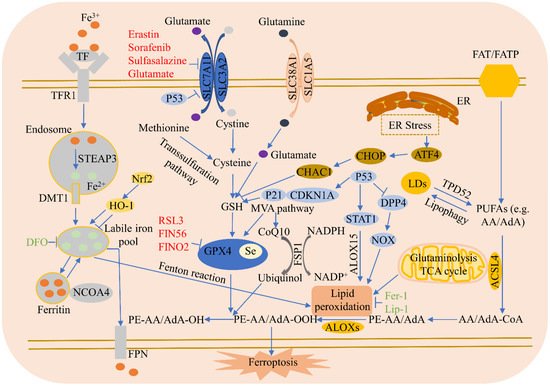
Ferroptosis regulatory pathways are shown in Figure 1.
Figure 1. Ferroptosis regulatory pathways. Ferroptosis regulatory pathways can be roughly classified into three types: The first involve iron metabolism, including the nuclear receptor coactivator 4 (NCOA4) regulation of ferritin degradation, and the Nrf2-HO-1 pathway, which affects iron. The second is the GSH/GPX4 pathway, including system Xc- inhibition, the transsulfuration pathway, mevalonate pathway (MVA pathway), glutamine pathway, and p53. The third type is that of lipid metabolism, including ACSL4, P53/SAT1/ALOX15, TPD52, and lipophagy, which are related to lipid regulation and ferroptosis, as well as the FSP1-CoQ10-NAD(P)H pathway synergies with GPX4 and GSH, which reduce phospholipid peroxidation and ferroptosis. In addition, endoplasmic reticulum (ER) stress facilitates ferroptosis via ATF4-induced CHAC 1 expression.
3.1. Iron Metabolism
Iron in food is principally Fe
3+, which combines with transferrin (TF) in serum and is then identified by TFR1 in the cell membrane. Once absorbed by TFR1, Fe
3+ is reduced to Fe
2+ through the STEAP3 metalloreductase in the endosome, which is subsequently released from the endosome into the cytosol via solute carrier family 11 member 2 (SLC11A2/DMT1) [
4]. The iron-storage protein ferritin plays an antiferroptosis role, including those of ferritin light chain and FTH1, and lysosomes can degrade ferritin to increase free iron levels. NCOA4-mediated ferritinophagy increases the degradation of ferritin by lysosomes, reduces iron storage, and promotes ferroptosis [
12]. Finally, the iron-efflux protein FPN exports iron out of cells. Blocking the iron release pathway in cell membranes increases sensitivity to ferroptosis.
3.2. Lipid Metabolism
The absorption of free PUFAs such as arachidonic acid (AA) or adrenoxyl (AdA) is mediated by fatty acid translocase (FAT) and fatty acid transport protein (FATP) [
13]. ACSL4 facilitates the incorporation of PUFAs into cell membranes and ferroptosis. Lipoxygenases (ALOXs) mediate lipid peroxidation and cause ferroptosis. Tumor protein D52 (TPD52)-mediated lipid storage represses RSL3-induced lipid peroxidation and succedent ferroptosis [
14]. Lipophagy, a process of autophagy, degrades lipid droplets (LDs), enhances the production of free fatty acids, and increases lipid peroxidation and ferroptosis [
14]. Sterol carrier protein 2 promotes the transportation of peroxidized lipids to mitochondria and accelerates GPX4 depletion-mediated ferroptosis. Finally, acetyl-CoA carboxylase alpha is involved in fatty acid β-oxidation and biosynthesis, and promotes ferroptosis.
3.3. Amino Acid Metabolism
Amino acid metabolism is closely related to ferroptosis regulation. Cysteine availability restricts GSH biosynthesis, while cysteine starvation induces GSH depletion and ferroptosis. When the available cysteine is limited, some cells utilize the transsulfuration pathway to transform methionine to cysteine [
15]. These cells do not require the cystine/glutamate antiporter system Xc- to import cystine and, therefore, are resistant to system Xc- inhibitor-induced ferroptosis. Glutamate is also an important regulator of ferroptosis. In high concentrations, it suppresses system Xc- and triggers ferroptosis. Glutamine degradation (via glutaminolysis) fuels the tricarboxylic acid (TCA) cycle and provides the basis for necessary biosynthetic processes, such as lipid biosynthesis. Cystine starvation and blocked cystine uptake cannot increase ROS accumulation, lipid peroxidation, and ferroptosis in the presence of glutamine deficiency or the inhibition of glutaminolysis [
16].
3.4. System Xc-
The amino acid antiporter system Xc- consists of two subunits: SLC7A11 and SLC3A2. System Xc- imports extracellular cystine into cells by exchanging intracellular glutamate. Erastin, sorafenib, sulfasalazine, and glutamate all inhibit SLC7A11 expression, causing cysteine deprivation, GSH deletion, and GPX4 inactivation to trigger ferroptosis [
17]. ATF3 enhances the ferroptosis induced by erastin via the repression of system Xc- [
18], while AMPK-mediated BECN1 phosphorylation increases ferroptosis by directly inhibiting system Xc- activity [
19]. Radiotherapy and immunotherapy enhance lipid oxidation and the ferroptosis of tumor cells by synergistically suppressing SLC7A11 [
20]. Sorafenib inhibits system Xc- function and induces ferroptosis [
21], while GDF15 knockdown facilitates the ferroptosis induced by erastin via the attenuation of SLC7A11 expression [
22]. Cardiac ferritin H deficiency reduces SLC7A11 expression and facilitates ferroptosis and cardiomyopathy [
23]. Nrf2 suppresses ferroptosis and reduces intestinal ischemia/reperfusion-induced acute lung injury (IIR-ALI) by increasing SLC7A11 and HO-1 [
24]. PARP inhibition increases ferroptosis by inhibiting SLC7A11 and cooperates with ferroptosis inducers in BRCA-proficient ovarian cancer [
25], while Nrf2 and STAT3 reduce ferroptosis by regulating SLC7A11, which alleviates IIR-ALI [
26].
3.5. GPX4
RSL3 represses GPX4 activity via covalent bonding with GPX4, resulting in the accumulation of lipid peroxides and, ultimately, ferroptosis. GSH is a co-factor in the catalysis of peroxides by GPX4 to produce alcohols; however, cysteine deprivation leads to GSH depletion, which directly inactivates GPX4, resulting in the subsequent induction of ferroptosis. Legumain has been shown to facilitate tubular ferroptosis via the promotion of chaperone-mediated GPX4 autophagy in acute kidney injury [
27], while the selenium-GPX4 axis reduces the ferroptosis of follicular helper T cells [
28]. Selenium is necessary for GPX4 to suppress hydroperoxide-induced ferroptosis [
29,
30]. FINO
2 promotes ferroptosis through GPX4 inactivation and iron oxidation [
31]. The downregulation of GPX4 during myocardial infarction triggers ferroptosis in cardiomyocytes [
32]. Dihydroartemisinin (DHA) induces ferroptosis in glioblastoma through the inhibition of GPX4 [
33]. SIRT3-activated autophagy promotes ferroptosis by increasing the AMPK/mTOR pathway and reducing GPX4 levels [
34]. GPX4 maintains Treg cell activation and reduces antitumor immunity by inhibiting lipid peroxidation and ferroptosis [
35]. Kaempferol protects from oxygen-glucose deprivation/reoxygenation-induced neuronal ferroptosis via the upregulation of the Nrf2/SLC7A11/GPX4 axis [
36]. Finally, the inactivation of GPX4 was found to trigger ferroptosis and acute renal failure in mice [
37].
3.6. Ferroptosis Suppressor Protein 1 (FSP1)
FSP1 suppresses ferroptosis independent of GSH. Under nicotinamide adenine dinucleotide phosphate (NADPH), FSP1 reduces ubiquinone, also called coenzyme Q10 (CoQ10), to ubiquinol, which can reduce lipid peroxidation and succedent ferroptosis. The FSP1-CoQ10-NAD(P)H pathway has been shown to synergize with GPX4 and GSH to repress phospholipid peroxidation and ferroptosis [
38], while a small molecule, NPD4928, reportedly enhances ferroptosis via the inhibition of FSP1 [
39]. Plasma-activated medium promotes ferroptosis by decreasing FSP1 expression in human lung cancer cells [
40]. Mesenchymal stem cell derived exosomes inhibit neuron ferroptosis by lncGm36569/microRNA (miR)-5627-5p/FSP1 axis in acute spinal cord injury [
41], and MiR-672-3p inhibition facilitates neural recovery via the suppression of ferroptosis by FSP1 [
42].
3.7. P53
P53 is a tumor-suppressor gene that plays a dual role in ferroptosis, regulating it via either a transcriptional or post-translational mechanism. P53 decreases cystine absorption by transcriptionally suppressing SLC7A11 expression, reduces intracellular GSH, and induces ferroptosis in tumor cells [
43]. P53 also enhances ferroptosis via the transcriptional induction of SAT1 or GSL2 [
44]. On the other hand, p53 inhibits ferroptosis by transcriptionally inducing CDKN1A/p21 (cyclin-dependent kinase inhibitor 1 A) expression [
45]. P53 also limits erastin-induced ferroptosis by blocking dipeptidyl peptidase 4 (DPP4) activity via a transcription-independent mechanism [
46].
3.8. Heme Oxygenase-1 (HO-1)
HO-1 plays a dual role in ferroptosis induction, associated with both the environment and cell type. HO-1 promotes the ferroptosis induced by erastin in HT-1080 fibrosarcoma cells [
47], while tagitinin C promotes colorectal cancer cell ferroptosis by activating the PERK-Nrf2-HO-1 signaling pathway [
48]. Ferroptosis inhibition effectively reduces dextran sulfate sodium (DSS)-induced ulcerative colitis, related to Nrf2/HO-1 signaling pathway blocking [
49]. However, HO-1 also functions as a negative regulator of ferroptosis, and cetuximab enhances RSL3-induced ferroptosis in KRAS mutant colorectal cancer cells through the inhibition of the Nrf2/HO-1 pathway [
50]. Gastrodin alleviates the ferroptosis of HT-22 cells triggered by glutamate by up-regulating the Nrf2/HO-1 signaling pathway [
51]. HO-1 plays a key antiferroptotic role in renal epithelial cells [
52]. Fraxetin decreases myocardial-infarction-mediated ferroptosis through AKT/Nrf2/HO-1 pathway activation [
53]. MiR-3587 inhibitor promotes HO-1 up-regulation, thereby protecting renal tissues from ischemia/reperfusion-induced ferroptosis [
54]. Melatonin reduces the level of ferroptosis by activating the Nrf2/HO-1signaling pathway in type 2 diabetic osteoporosis [
55]. Panaxydol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via reducing ferroptosis by upregulating the Keap1-Nrf2/HO-1 pathway [
56]. Ginkgetin, which is derived from
Ginkgo biloba leaves, increases cisplatin-induced ferroptosis via the disruption of the Nrf2/HO-1 axis [
57].
3.9. NCOA4
Recent studies have shown that ferritinophagy, a selective autophagy, can degrade ferritin. NCOA4, a selective cargo receptor, transports ferritin to lysosomes where it is then degraded, then labile iron is released and oxygen radicals are increased to induce ferroptosis [
12]. NCOA4-mediated ferritinophagy enhances erastin-induced ferroptosis in HeLa cells [
58]. The loss of coatomer protein complex subunit zeta 1 increases NCOA4 expression and the ferroptosis of glioblastoma cell lines [
59]. DNA (cytosine-5)-methyltransferase 1 inhibition reduces ferroptosis via NCOA4-mediated ferritinophagy during diabetes myocardial ischemia/reperfusion injury [
60]. Formosanin C promotes the ferroptosis of human hepatocellular carcinoma cells by elevating NCOA4-mediated ferritinophagy [
61]. Disrupting NCOA4-FTH1 interaction inhibits ferroptosis [
62]. NCOA4-mediated ferritinophagy increases the ferroptosis induced by cerebral ischemia [
63].
3.10. Endoplasmic Reticulum (ER) Stress
ER stress is triggered under different pathological conditions and is closely associated with the course of cell death. Erastin can induce ER stress and up-regulates ER-stress-responsive genes [
21]. The eif2α-ATF4 axis is the ferroptotic reagent-activated primary signaling pathway. CHAC 1 is downstream of ATF4 and degrades GSH while promoting the succedent ferroptosis [
64].
3.11. Common Inducers and Inhibitors of Ferroptosis
The four known mechanisms of ferroptosis inducers are: (1) the inhibition of system Xc- and depletion of intracellular GSH by, for example, erastin, sorafenib, sulfasalazine, or glutamate; (2) the inhibition of GPX4 by, for example, RSL3, which covalently combines with GPX4 to reduce its activity, resulting in the accumulation of toxic lipid peroxides and, ultimately, ferroptosis; (3) the degradation of GPX4 and exhaustion of antioxidant CoQ10 by, for example, FIN56; and (4) the direct oxidation of ferrous iron and lipidome, or indirect inactivation of GPX4 by, for example, FINO
2 [
31]. There are two mechanisms of ferroptosis inhibitors: (1) inhibiting the accumulation of iron, such as that of deferoxamine (DFO), which chelates iron and limits the Fenton reaction to prevent lipid peroxidation; and (2) the inhibition of lipid peroxidation by, for example, ferrostatin-1 (Fer-1), liproxststatin-1 (Lip-1), and vitamin E, which act as radical scavengers to reduce lipid peroxides and effectively block ferroptosis. DFO is commercially available as Desferal, an iron chelator clinically approved for the treatment of acute iron intoxication and chronic iron overload [
65]. For the action mechanism, Desferal is a hexadentate molecule that is able to bind free plasma iron and excess iron within cells at a 1-to-1 ratio and then is excreted via the urine or bile [
66].