The genus mint (Mentha) belongs to the Lamiaceae family and includes 42 species, 15 hybrids, and hundreds of subspecies, varieties, and cultivars, which potentially crossbreed when in proximity. Different mints are known for a reasonably high content of essential oils (EO), which are deposited in the glandular trichomes, mostly located on the adaxial surface of their leaves. There are two well-known, so-called menthol mints in cultivation: Mentha x piperita L. (Hudson): peppermint—MP, and Mentha arvensis L., (syn. M. canadensis L., Japanese mint): cornmint—MA.
- botanical pesticides
- chemical composition
- agriculture
1. Introduction
2. Content and Chemical Composition of Peppermint Oil and Cornmint Oil
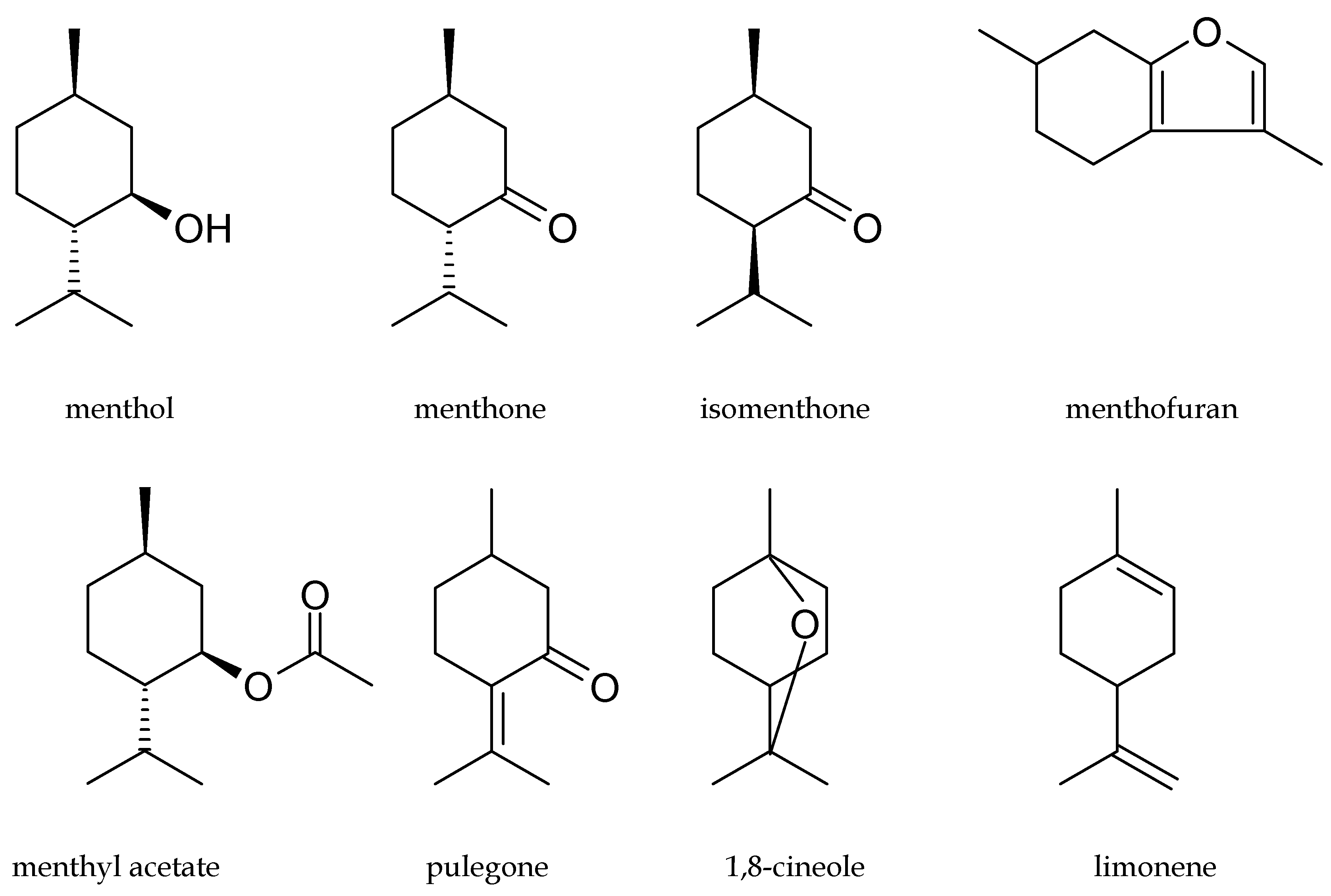
3. Biological Activity and Application of Peppermint Oil and Cornmint Oil
4. Antifungal and Antibacterial Activity of Peppermint Oil and Cornmint Oil against Phytopathogens
|
Fungi/Bacteria (B) |
MIC or Total Inhibition Concentration |
No. of Essential Oils Mint Oil Composition [%] |
Methods Results for the Most Active Essential Oil |
Ref. |
|---|---|---|---|---|
|
Alternaria alternata Alternaria solani Aspergillus flavus Aspergillus niger Fusarium solani Rhizopus solani Rhizopus spp. |
117.0/57.9μg/mL1 127.1/129.0 122.0/110.7 49.5/63.5 130.7/89.8 44.11/63.9 149.7/137.1 |
4 EOs, 4 compounds MPEO menthone 28.1, menthol 4.8, menthyl acetate 9.5, limonene 7.1 MAEO, menthol 78.9, menthone 6.4 |
broth microdilution, agar disc diffusion (15 μL), positive control: fluconazole 30 μg M. spicata and M. longifolia similar results as MPEO |
[39] |
|
Alternaria brassicae Botrytis cinerea Cladobotryum mycophilum Fusarium oxysporum Phytophthora parasitica Pythium aphanidermatum Sclerotinia sclerotiorumisolated from vegetables and mushrooms |
16.2%2 - 7.4% 15.8% 5.7% - 6% |
12 EOs MPEO menthol 42.0, menthone 28.8, 1,8-cineole 7.1 |
disc diffusion 8 μL of 5–30% EO solution MPEO oil belonged to four most effective |
[40] |
|
Alternaria citrii Aspergillus fumigatus Aspergillus oryzae Fusarium oxysporum Fusarium solani Helminthosporium compactum Macrophomina phaseolina Sclerotium rolfsii |
0.25 μL/mL 1.0 1.0 3.0 2.0 0.5 2.0 0.25 |
4 EOs MPEO no data |
disc diffusion, 5 μL, agar dilution 0.16–20 μg/mL MPEO less active than other three |
[41] |
|
Alternaria citrii Botrytis cinerea Colletotrichum gloeosporioides Lasiodiplodia theobromae Penicillium digitatum isolated from fruits |
3000 μL/L 3000 3000 >3000 2000 |
18 EOs MPEO menthol 40.7, menthone 21.7 |
agar dilution thyme 500–1000 μL/L (3000 P. digitatum) |
[42] |
|
Aspergillus ochraceus |
2000 μg/L (broth) 1500 μg/L (vapor) |
5 EOs, 5 compounds MPEO menthol 50 |
broth dilution/vapor phase cinnamon oil and cinnamaldehyde: 250–500 μg/L (broth), 150–250 μg/L (vapor), ochratoxin A production inhibited at 200 μg/L |
[43] |
|
Aspergillus ochraceus |
1000 ppm |
4 EOs MAEO, no data |
broth dilution MAEO and oregano oil were the most effective in inhibition of fungal growth and ochratoxin A production |
[44] |
|
Aspergillus flavus Aspergillus niger Aspergillus parasiticus Penicillium chrysogenum |
10000 ppm 5000 2500 1250 |
8 EOs and EOs combinations MPEO menthol, menthone |
broth dilution, vapor phase MPEO less active than thyme (312.5–1250 ppm) and oregano oils, similar activity to cinnamon oil, more active than other four oils |
[45] |
|
Aspergillus flavus Aspergillus niger Fusarium oxysporum Mucor spp. Penicillium digitatum |
1.13/2.25 mg/mL3 1.13/2.25 1.13/2.25 1.13/2.25 2.25/4.5 |
MPEO |
agar dilution (MIC), broth dilution (MFC), well diffusion, vapor phase |
[46] |
|
Aspergillus flavus Aspergillus fumigatus Aspergillus niger Botryodiplodia theobromae Cladosporium cladosporioides Fusarium oxysporum Helminthosporium oryzae Macrophomina sp. Sclerotium rolfsii |
0.1 mg/mL 0.1 <0.5 0.1 0.1 |
18 EOs MAEO menthol 73, menthone 6.1 |
agar dilution, positive control: four synthetic fungicides MAEO was the most efficient of EOs and more efficient than synthetic fungicides at 0.1 mg/mL four fungi were inhibited totally, other 72–100% inhibition aflatoxin B1 production by A. flavus inhibited at 0.05 mg/mL |
[47] |
|
Alternaria alternata Aspergillus fumigatus Aspergillus candidus Aspergillus nidulans Aspergillus versicolor Cladosporium cladosporioides Curvularia lunata Fusarium nivale Fusarium oxysporum Fusarium roseum Penicillium sp. Monilia sp. Trichoderma viride |
400 μg/L |
18 EOs MAEO no data |
agar dilution, positive control: nine synthetic fungicides MAEO was the most efficient of EOs and more efficient than all synthetic fungicides at 400 μg/L 11 fungi were inhibited totally, other two >84% |
[48] |
|
Botrytis cinerea Geotrichum citri-aurantii Phytophthora citrophthora Penicillium digitatum |
no inhibition at 250 ppm |
19 EOs MPEO menthol 50, menthone 30, menthyl acetate 10 |
radial growth on plate at different concentration, positive control: four synthetic fungicides Chrysanthemum viscidehirtum total inhibition at 150 ppm, synthetic fungicides at 50 ppm |
[49] |
|
Phytophthora cinnamomi Pyrenochaeta lycoprsici Verticillium dahliae |
800 ppm 400 800 |
8 EOs MPEO menthol 39.0, menthone 21.0, menthofuran 19.5, 1,8-cineole 7.0 |
agar dilution oregano 200, 50, 50 ppm, resp. |
[50] |
|
Dreschlera spicifera Fusarium oxysporum f.sp. ciceris Macrophomina phaseolina |
1600 ppm >1600 800 |
MPEO menthol 25.2, menthone 30.6 |
agar dilution |
[51] |
|
Colletotrichum gloeosporioides isolated from fruits |
2.0 mg/mL |
28 EOs MPEO, no data |
agar microdilution, positive control: amphotericin B 5–60 μL/mL coriander leaf, two lemongrass sp. 0.25 mg/mL (lemongrass oil evaluated on passion fruit) |
[52] |
|
Fusarium spp. Penicillium spp. Phythium spp. isolated from corn seeds |
1000 μL/L 1000 >1000 |
18 EOs MPEO, no data |
agar dilution oregano MIC 100–200 μL/L |
[53] |
|
Mucor sp. Rhizopus stolonifer Sclerotinia sclerotiorum |
30 μL/400 mL air |
2 EOs, 4 compounds MPEO menthol 33.3, menthone 29.5, 1,8-cineole 7.0 |
vapor phase sweet basil and menthol 30 μL/400 mL air, menthone not active |
[54] |
|
Rhizoctonia botaticola Sclerotium rolfsii |
1000 μg/mL |
20 EOs MAEO, no data |
agar dilution 6 EOs totally inhibited both fungi’s growth at 1000 μg/mL |
[55] |
|
Lecanicillium fungicola var. fungicola |
750–1000 μL/L |
11 EOs MPEO menthol 39.2, menthyl acetate 20.4, menthone 15.3 |
broth dilution mushroom Agaricus bisporus MPEO was similarly active against mushroom and its pest, savory and thyme oils showed the best selectivity index |
[56] |
|
Aspergillus niger Penicillium funiculosum |
11.4 μg/mL 11.4 |
9 EOs MPEO linalool 41.4 linalyl ac 39.5 |
agar dilution Thymus letrobotrys 2.7 and 2.2 μg/mL |
[57] |
|
Alternaria alternata Aspergillus flavus Aspergillus fumigatus Cladosporium herbarum Fusarium oxysporum Aspergillus veriscolor Fusarium acuminatum Fusarium solani Fusarium tabacinum Monilinia fructicola Penicilliumspp. Rhizoctonia solani Sclerotinia minor Sclerotinia sclerotiorum (B) Pseudomonas syringae (B) Xanthomonas campestris |
1.50 μg/mL 10.0 0.50 1.50 1.50 10.0 2.50 10.0 1.50 5.50 1.50 1.50 10.0 10.0 2.50 80.0 |
MPEO menthol 36.0, isomenthone 23.5, menthone 24.6, menthyl acetate 9.0, menthofuran 6.9 |
disc diffusion 10 μL, broth microdilution, positive control: amphotericin B MIC 1–5 μg/mL menthol, menthone MIC against P. syringae 2.0, 1.0 μg/mL X. campestris 2.0, 2.0 μg/mL |
[58] |
|
Alternaria alternata Aspergillus flavus Aspergillus niger Aspergillus ochraceus Aspergillus terreus Aspergillus versicolor Cladosporium cladosporioides Fusarium tricinctum Penicillium funiculosum Penicillium ochrochloron |
1.5–3.0 μL/mL in ethanol 1.0–2.5 μL/mL in Tween |
4 EOs MPEO menthol 37.4, menthone 12.7, limonene 6.9, menthofuran 6.8 |
agar macro- (in ethanol) and micro- (in Tween) dilution, positive control: bifonazol MIC 10–15 μL/mL thyme oil 0.125–0.5 μL/mL in ethanol, 0.05–0.25 in Tween menthol 0.25–1.5 μL/mL in ethanol, 0.05–1.0 μL/mL in Tween |
[59] |
|
Trichoderma harzianum Verticillium fungicola (B) Pseudomonas tolaasii |
3–4 μL/mL |
10 EOs, 10 compounds MPEO menthol 37.4, menthyl acetate 17.4, menthone 12.7 |
microdilution, macrodilution, disc diffusion, vapor phase, positive control: bifonazol and prochloraz (fungi), streptomycin + penicillin (bacteria) oregano and thyme 1.5–2.0 μL/mL |
[60] |
|
(B) Agrobacterium tumefaciens (B) Erwinia carotovora |
13 EOs, 14 compounds MPEO, no data |
agar diffusion, 50 μL solution MPEO moderately active at 200 mg/mL 6 EOs were effective, MPEO showed weak activity |
[61] |
|
|
Aspergillus flavus Aspergillus parasiticus Fusarium solani Sclerotium rolfsii (B) Pseudomonas syringae pv. phaseolicola (B) Pseudomonas syringae pv. tomato (B) Pseudomonas syringae pv. syringae (B) Xanthomonas campestris pv. campestris (B) Xanthomonas campestris pv. phaseoli |
- - - - 0.07–0.625 mg/mL 0.156–0.312 0.156–0.312 0.312–0.625 0.625–2.5 |
four MPEO menthol 27.5–42.3, menthone 18.4–27.9 |
fungi: agar diffusion, 50 μL, weak activity bacteria: microdilution menthol 0.07–1.25 mg/mL menthone 1.25–2.5 mg/mL |
[62] |
This entry is adapted from the peer-reviewed paper 10.3390/molecules25010059
References
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of Genus Mentha: From Farm to Food Factory. Plants 2018, 7, 70.
- Tiwari, P. Recent advances and challenges in trichome research and essential oil biosynthesis in Mentha arvensis L. Ind. Crop. Prod. 2016, 82, 141–148.
- Pushpangadan, P.; Tewari, S.K. Peppermint. In Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2006; pp. 460–481.
- Bacon, F.J. The Botanical Origin of American Peppermint—Mentha Piperita L*. J. Am. Pharm. Assoc. (1912) 1928, 17, 1094–1096.
- Maffei, M.; Sacco, T. Chemical and Morphometrical Comparison Between two Peppermint Notomorphs. Planta Medica 1987, 53, 214–216.
- Grzeszczuk, M.; Jadczak, D. Estimation of biological value of some species of mint (Mentha L.). Herba Polonica. 2009, 55, 193–199.
- Machiani, M.A.; Javanmard, A.; Morshedloo, M.R.; Maggi, F. Evaluation of competition, essential oil quality and quantity of peppermint intercropped with soybean. Ind. Crop. Prod. 2018, 111, 743–754.
- Machiani, M.A.; Javanmard, A.; Morshedloo, M.R.; Maggi, F. Evaluation of yield, essential oil content and compositions of peppermint (Mentha piperita L.) intercropped with faba bean (Vicia faba L.). J. Clean. Prod. 2018, 171, 529–537.
- Ulbrich, A.; Kahle, H.; Kramer, P.; Schulz, M. Mentha x piperita volatiles promote Brassica oleracea-A pilot study for sustainable vegetable production. Allelopath. J. 2018, 43, 93–104.
- Karkanis, A.; Alexiou, A.; Katsaros, C.; Petropoulos, S. Allelopathic Activity of Spearmint (Mentha spicata L.) and Peppermint (Mentha × piperita L.) Reduces Yield, Growth, and Photosynthetic Rate in a Succeeding Crop of Maize (Zea mays L.). Agron. 2019, 9, 461.
- Karamaouna, F.; Kimbaris, A.; Michaelakis, Α.; Papachristos, D.; Polissiou, M.; Papatsakona, P.; Tsora, E. Insecticidal Activity of Plant Essential Oils Against the Vine Mealybug, Planococcus ficus. J. Insect Sci. 2013, 13, 1–13.
- Ram, M.; Kumar, S. Yield and Resource Use Optimization in Late Transplanted Mint (Mentha arvensis) under Subtropical Conditions. J. Agron. Crop. Sci. 1998, 180, 109–112.
- Akram, M.; Uzair, M.; Malik, N.S.; Mahmood, A.; Sarwer, N.; Madni, A.; Asif, H.M. Mentha arvensis Linn: A review article. J. Medic. Plants Res. 2011, 5, 4499–4503.
- Singh, M.; Singh, A.; Singh, S.; Tripathi, R.S.; Singh, A.K.; Patra, D.D. Cowpea (Vigna unguiculata L. Walp.) as a green manure to improve the productivity of a menthol mint (Mentha arvensis L.) intercropping system. Ind. Crops Prod. 2010, 31, 289–293.
- Chand, S.; Patra, N.K.; Anwar, M.; Patra, D.D. Agronomy and uses of menthol mint (Mentha arvensis)—Indian perspective. Proc. Indian. Natl. Sci. Acad. 2004, 70, 269–297.
- Costa, A.; Chagas, J.; Bertolucci, S.; Pinto, J. Growth and production of volatiles in fertilized mint. Acta Hortic. 2018, 63–66.
- Adamović, D.S.; Đalović, I.G.; Mrkovački, N.M.; Pandurevic, T.; Bjelic, D.; Tyr, S. Effect of growing season upon microbial status of peppermint (Mentha x piperita L.) rhizosphere. Acta Fytotech. Zootech. 2015, 18, 99–102.
- Mousavinik, S.M.; Asgharipour, M.R.; Sardashti, S. Manure and Light Intensity Affect Growth Characteristics and Essential Oil of Peppermint (Mentha piperita L.). J. Essent. Oil Bear. Plants 2016, 19, 2029–2036.
- Bajeli, J.; Tripathi, S.; Kumar, A.; Tripathi, A.; Upadhyay, R. Organic manures a convincing source for quality production of Japanese mint (Mentha arvensis L.). Ind. Crop. Prod. 2016, 83, 603–606.
- Verma, R.K.; Verma, R.S.; Rahman, L.-U.; Kalra, A.; Patra, D.D. Integrated Nutrient Management on Biomass, Oil Yields and Essential Oil Composition of Peppermint (Mentha piperita L.) and Residual Fertility in a Hilly Soil. J. Essent. Oil Bear. Plants 2016, 19, 582–591.
- USEPA (U.S. Environmental Protection Agency). Exemption of certain pesticide substances from federal insecticide, fungicide, and rodenticide act requirements, Washington DC, USA. 1996. Available online: http://www.nj.gov/dep/enforcement/pcp/bpo/min_risk.pdf (accessed on 23 December 2019).
- Isman, M.B. Botanical Insecticides in the Twenty-First Century-Fulfilling Their Promise? Annu. Rev. Èntomol. 2019, 65, 65.
- Benelli, G.; Pavela, R.; Maggi, F.; Petrelli, R.; Nicoletti, M. Commentary: making green pesticides greener? The potential of plant products for nanosynthesis and pest control. J. Cluster Sci. 2017, 28, 3–10.
- Isenring, R. Pesticides and the loss of biodiversity; Pesticide Action Network Europe: London, UK, 2010.
- Maino, J.L.; Binns, M.; Umina, P. No longer a west-side story – pesticide resistance discovered in the eastern range of a major Australian crop pest, Halotydeus destructor (Acari: Penthaleidae). Crop. Pasture Sci. 2018, 69, 216.
- Balakrishnan, A. Therapeutic uses of peppermint—a review. J. Pharm. Sci. Res. 2015, 7, 474–476.
- Varshney, S.C. Indian mint oils. Perf. Flav. 2005, 30, 36–44.
- European Pharmacopoeia 5.0, 5.1–5.8. Strasbourg Council of Europe. 2005.
- Lawrence, B.M. Progress in essential oils. Perf. Flav. 1993, 18, 59–71.
- Telci, I.; Kacar, O.; Bayram, E.; Arabacı, O.; Demirtaş, I.; Yılmaz, G.; Özcan, I.; Sönmez, Ç.; Göksu, E. The effect of ecological conditions on yield and quality traits of selected peppermint (Mentha piperita L.) clones. Ind. Crop. Prod. 2011, 34, 1193–1197.
- Oroian, C.; Covrig, I.; Odagiu, A.; Mălinaș, C.; Moldovan, C.; Fleșeriu, A. Effects of Cultivation Systems and Environmental Conditions on Peppermint (Mentha × piperita L.) Biomass Yield and Oil Content. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 576–581.
- Santoro, M.V.; Bogino, P.C.; Nocelli, N.; Cappellari, L.D.R.; Giordano, W.F.; Banchio, E. Analysis of Plant Growth-Promoting Effects of Fluorescent Pseudomonas Strains Isolated from Mentha piperita Rhizosphere and Effects of Their Volatile Organic Compounds on Essential Oil Composition. Front. Microbiol. 2016, 7, 11.
- Shahabivand, S.; Padash, A.; Aghaee, A.; Nasiri, Y.; Rezaei, P.F. Plant biostimulants (Funneliformis mosseae and humic substances) rather than chemical fertilizer improved biochemical responses in peppermint. Iran. J. Plant Physiol. 2018, 8, 2333–2344.
- Shaikh, M.N.; Kasabe, U.I.; Mokat, D.N. Influence of Rhizosphere Fungi on Essential Oil Production and Menthol Content in Mentha arvensis L. J. Essent. Oil Bear. Plants 2018, 21, 1076–1081.
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 45.
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmet. 2018, 5, 11.
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829.
- Van de Vel, E.; Sampers, I.; Raes, K. A review on influencing factors on the minimum inhibitory concentration of essential oils. Crit. Rev. Food Sci. Nutrit. 2019, 59, 357–378.
- Hussain, A.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836.
- Diánez, F.; Santos, M.; Parra, C.; Navarro, M.; Blanco, R.; Gea, F. Screening of antifungal activity of 12 essential oils against eight pathogenic fungi of vegetables and mushroom. Lett. Appl. Microbiol. 2018, 67, 400–410.
- Pattnaik, S.; Subramanyam, V.R.; Kole, C. Antibacterial and antifungal activity of ten essential oils in vitro. Microbios 1996, 86, 237–246.
- Combrinck, S.; Regnier, T.; Kamatou, G. In vitro activity of eighteen essential oils and some major components against common postharvest fungal pathogens of fruit. Ind. Crop. Prod. 2011, 33, 344–349.
- Hua, H.; Xing, F.; Selvaraj, J.N.; Wang, Y.; Zhao, Y.; Zhou, L.; Liu, X.; Liu, Y. Inhibitory Effect of Essential Oils on Aspergillus ochraceus Growth and Ochratoxin A Production. PLOS ONE 2014, 9, e108285.
- Basílico, M.Z. Inhibitory effects of some spice essential oils on Aspergillus ochraceus NRRL 3174 growth and ochratoxin A production. Lett. Appl. Microbiol. 1999, 29, 238–241.
- Hossain, F.; Follett, P.; Vu, K.D.; Harich, M.; Salmieri, S.; Lacroix, M. Evidence for synergistic activity of plant-derived essential oils against fungal pathogens of food. Food Microbiol. 2016, 53, 24–30.
- Tyagi, A.K.; Malik, A. Antimicrobial potential and chemical composition of Mentha piperita oil in liquid and vapour phase against food spoiling microorganisms. Food Control. 2011, 22, 1707–1714.
- Kumar, R.; Dubey, N.K.; Tiwari, O.P.; Tripathi, Y.B.; Sinha, K.K. Evaluation of some essential oils as botanical fungitoxicants for the protection of stored food commodities from fungal infestation. J. Sci. Food Agric. 2007, 87, 1737–1742.
- Kumar, A.; Shukla, R.; Singh, P.; Singh, A.K.; Dubey, N.K. Use of essential oil from Mentha arvensis L. to control storage moulds and insects in stored chickpea. J. Sci. Food Agric. 2009, 89, 2643–2649.
- Bouchra, C.; Mohamed, A.; Hassani Mina, I.; Hmamouchi, M. Antifungal activity of essential oils from several medicinal plants against four postharvest citrus pathogens. Phytopathol. Mediterr. 2003, 42, 251–256.
- Giamperi, L.; Fraternale, D.; Ricci, D. The In Vitro Action of Essential Oils on Different Organisms. J. Essent. Oil Res. 2002, 14, 312–318.
- Moghaddam, M.; Pourbaige, M.; Tabar, H.K.; Farhadi, N.; Hosseini, S.M.A. Composition and Antifungal Activity of Peppermint (Mentha piperita) Essential Oil from Iran. J. Essent. Oil Bear. Plants 2013, 16, 506–512.
- Anaruma, N.D.; Schmidt, F.L.; Duarte, M.C.T.; Figueira, G.M.; Delarmelina, C.; Benato, E.A.; Sartoratto, A. Control of Colletotrichum gloeosporioides (Penz.) Sacc. In yellow passion fruit using Cymbopogon citratus essential oil. Braz. J. Microbiol. 2010, 41, 66–73.
- Christian, E.J.; Goggi, A.S. Aromatic Plant Oils as Fungicide for Organic Corn Production. Crop. Sci. 2008, 48, 1941.
- Edris, A.E.; Farrag, E.S. Antifungal activity of peppermint and sweet basil essential oils and their major aroma constituents on some plant pathogenic fungi from the vapor phase. Food/Nahrung 2003, 47, 117–121.
- Sharma, P.K.; Raina, A.P.; Dureja, P. Evaluation of the antifungal and phytotoxic effects of various essential oils against Sclerotium rolfsii (Sacc) and Rhizoctonia bataticola (Taub). Arch. Phytopathol. Plant Prot. 2009, 42, 65–72.
- Mehrparvar, M.; Goltapeh, E.M.; Safaie, N.; Ashkani, S.; Hedesh, R.M. Antifungal activity of essential oils against mycelial growth of Lecanicillium fungicola var. fungicola and Agaricus bisporus. Ind. Crop. Prod. 2016, 84, 391–398.
- El Asbahani, A.; Jilale, A.; Voisin, S.N.; Aït Addi, E.H.; Casabianca, H.; El Mousadik, A.; Hartmann, D.J.; Renaud, F.N. Chemical composition and antimicrobial activity of nine essential oils obtained by steam distillation of plants from the Souss-Massa Re Region (Morocco). J. Essent. Oil Res. 2015, 27, 34–44.
- Reddy, D.N.; Al-Rajab, A.J.; Sharma, M.; Moses, M.M.; Reddy, G.R.; Albratty, M. Chemical constituents, in vitro antibacterial and antifungal activity of Mentha piperita L. (peppermint) essential oils. J. King Saud Univ. Sci. 2017.
- Soković, M.D.; Vukojevic, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; Van Griensven, L.J.L.D. Chemical Composition of Essential Oils of Thymus and Mentha Species and Their Antifungal Activities. Molecules 2009, 14, 238–249.
- Sokovic, M.; Van Griensven, L.J. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224.
- El-Zemity, S.R.; Radwan, M.A.; El-Monam Mohamed, S.A.; Sherby, S.M. Antibacterial screening of some essential oils, monoterpenoids and novel N-methyl carbamates based on monoterpenoids against Agrobacterium tumefaciens and Erwinia carotovora. Arch. Phytopathol. Plant Prot. 2008, 41, 451–461.
- Işcan, G.; Kïrïmer, N.; Kürkcüoǧlu, M.; Başer, H.C.; Demïrcï, F. Antimicrobial Screening of Mentha piperita Essential Oils. J. Agric. Food Chem. 2002, 50, 3943–3946.
- Choi, O.; Cho, S.K.; Kim, J.; Park, C.G.; Kim, J. Antibacterial properties and major bioactive components of Mentha piperita essential oils against bacterial fruit blotch of watermelon. Arch. Phytopathol. Plant Prot. 2016, 49, 325–334.
- Lis-Balchin, M.; Deans, S.G.; Eaglesham, E. Relationship between bioactivity and chemical composition of commercial essential oils. Flavour Fragr. J. 1998, 13, 98–104.
- Chao, S.C.; Young, D.G.; Oberg, C.J. Screening for Inhibitory Activity of Essential Oils on Selected Bacteria, Fungi and Viruses. J. Essent. Oil Res. 2000, 12, 639–649.
- Baruah, P.; Sharma, R.K.; Singh, R.S.; Ghosh, A.C. Fungicidal Activity of Some Naturally Occurring Essential Oils Against Fusarium moniliforme. J. Essent. Oil Res. 1996, 8, 411–412.
- Ahmad, A.; Darokar, M.P.; Tandon, S. Antimicrobial activity of isolates and derivatives of Indian Mentha arvensis essential oil. J. Essent. Oil Bear. Plants 2011, 14, 796–804.
- Montes-Belmont, R.; Carvajal, M. Control of Aspergillus flavus in maize with plant essential oils and their components. J. Food Prot. 1998, 61, 616–619.
- Tsao, R.; Zhou, T. Antifungal activity of monoterpenoids against postharvesr pathogens Botrytis cinerea and Monilinia fructicola. J. Essent. Oil Res. 2000, 12, 113–121.
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R.; Abbaszadeh, A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. Journal de Mycologie Médicale 2014, 24, e51–e56.
- Kotan, R.; Kordali, S.; Cakir, A. Screening of antibacterial activities of twenty-one oxygenated monoterpenes. Zeitschrift für Naturforschung C 2007, 62, 507–513.
- Vieira-Brock, P.L.; Vaughan, B.M.; Vollmer, D.L. Comparison of antimicrobial activities of natural essential oils and synthetic fragrances against selected environmental pathogens. Biochim. Open 2017, 5, 8–13.
