Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Many obesity and diet-related diseases have been observed. Insulin resistance (IR), a state of tissue resistance to insulin due to its impaired function, is a common coexisting condition. The most important predisposing factors are excessive visceral fat and chronic low-grade inflammatory response. An additional disease that is often associated with IR is urolithiasis. The common feature of these two conditions is metabolic acidosis and mild inflammation. A patient diagnosed with IR and urolithiasis is a big challenge for a dietitian.
- insulin
- insulin resistance
- diet
- dietitian
- urolithiasis
1. Introduction
The advancement of technology, the increasing availability of caloric fast food, a decrease in society’s physical activity, and a sedentary lifestyle have led to a significant increase in society’s average weight [1]. The prevalence of obesity has increased worldwide over the past 50 years, reaching a pandemic level [2]. Along with civilisation’s progress, obesity-related diseases such as type 2 diabetes, hypertension, lipid disorders, and urolithiasis are increasing [3][4]. Tissue resistance to insulin is a common condition that coexists with these diseases. The pathogenesis of insulin resistance (IR) is not yet fully understood. The studies suggest that mutations, excessive visceral fat, chronic low-grade inflammation, and prolonged excessive sympathetic stimulation may be predisposing factors [5][6][7][8]. A properly selected and balanced diet may help increase tissue sensitivity to insulin, reduce the risk of many diseases, and achieve the desired body weight value [9]. Due to disturbed fat metabolism and abnormal carbohydrate metabolism found in IR, a patient with this disorder often challenges a dietitian. The association of IR with urolithiasis seems to be even greater.
2. Insulin Resistance
IR is a condition of impaired insulin action by which glucose homeostasis is disturbed. As a result, tissue response to insulin is reduced while maintaining normal or elevated blood levels. Resistant tissues cannot properly metabolise glucose that remains in the blood. As a result, pancreatic β cells do not stop producing insulin. Excessive insulin synthesis leads to increased tissue resistance to insulin, forming a vicious circle. A simplified diagram of the vicious circle is shown in Figure 1. The chronic inflammatory response and low activity cause IR development [5][6][10][11][12]. The reasons for IR include (1) insufficient physical activity, (2) visceral fat level, (3) impact of some types of drugs, and (4) genetic factors. Moreover, it has been discovered that the development of IR can be associated with several conditions, such as cardiovascular diseases, non-alcoholic fatty liver disease, strokes, or polycystic ovary syndrome. IR significantly increases the risk of type 2 diabetes and metabolic syndrome [12][13][14][15].
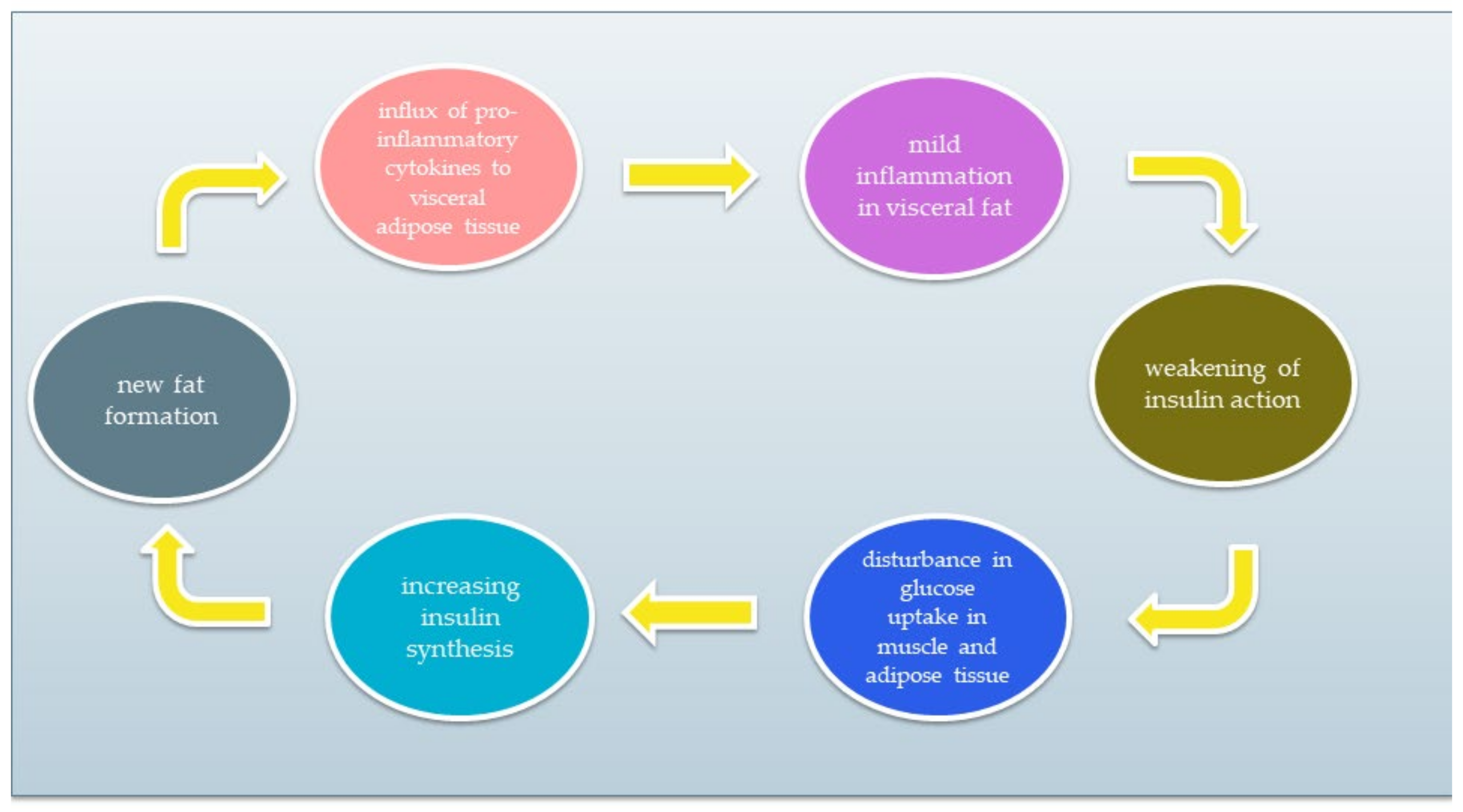
Figure 1. The vicious circle of insulin resistance.
There are two types of IR: hepatic and peripheral. Hepatic IR is characterised by increased glycogenolysis and gluconeogenesis in hepatocytes and increased very-low-density lipoprotein cholesterol (VLDL-C) and triglycerides synthesis. In turn, peripheral IR refers to skeletal muscle and fat. Then, glucose uptake by skeletal muscles is impaired, and fat distribution in adipose tissue increases, which leads to the release of free fatty acids (FFA) [16].
IR can also be divided into pre-receptor, receptor, and post-receptor disorders. The pre-receptor disease is characterised by abnormal insulin molecule structure. An example would be an endogenous insulin molecule’s genetically conditioned, abnormal structure. In this case, when the exogenous insulin is administered, glucose absorption into the cell is normal. Receptor IR occurs when a gene responsible for the insulin receptor’s structure or function is mutated. Mutations cause inappropriate binding of insulin to the receptor. Post-receptor IR occurs in the case of disorders of processes signalling the attachment of insulin to the insulin receptor or diseases of the structure and operation of glucose transporters to the cell [16].
3. Urolithiasis
Urolithiasis is one of the oldest known diseases. Through archaeological research of Egyptian mummies, it has been proven that this disorder affected society in antiquity. It is characterised by insoluble deposits (stones) in the urinary tract formed due to the crystallisation of components present in the urine [17]. These stones often cause discomfort in the form of acute colic pain, nausea, and vomiting. They range in size from a few micrometres to a few centimetres. It is estimated that about 97% is in the kidneys and ureters, and 3% is in the bladder and urethra [18]. Thanks to technological advancement, researchers now know that stones can consist of about 100 different chemical compounds and 80% of them mainly contain calcium oxalate (CaOx). Besides calcium oxalates, researchers usually find calcium phosphate and gout stones [19]. Treatment of urolithiasis usually involves surgical removal of the stones. Unfortunately, it does not remove the causes of their formation [18]. Scientific research indicates a relationship between the occurrence of urolithiasis with diet, lifestyle, and climate [18][19][20].
4. Urolithiasis, Metabolic Acid, Inflammation, and Insulin Resistance
The mechanisms linking urolithiasis to the features of MS are not fully understood. Nevertheless, attention is often paid to the mild inflammation characteristic of MS, which also occurs in the presence of IR [21]. One of the most frequently studied inflammatory markers is interleukin-8 (IL-8/CXCL8). Its role in the development of neoplasms is often emphasised [22]. Its secretion is stimulated by hypoxia, reactive oxygen species, bacterial particles, and other cytokines such as IL-1, IL-6, or TNFα. The cells that secrete it are mainly leukocytes: blood monocytes, macrophages, epithelial and endothelial cells, and fibroblasts [22][23]. Suen et al. [24] focused on finding prognostic markers of urolithiasis and showed that the presence of urinary stones is associated with an inflammatory response, especially with increased levels of IL-8 in the urine. They suggest that IL-8 could be a marker as a screening test for urolithiasis. Wang et al. [25] showed that the values of pro-inflammatory cytokines, including IL-8 in plasma, decreased in patients after percutaneous nephrolithotripsy and suggested that IL-8 can be used as a predictive tool for patients after this procedure.
Another proposed explanation is the association of low ammonium excretion with urine in MS patients, which causes acidification of the urine [20][26]. Systemic metabolic acidosis alters the concentration of various substances in the urine and may contribute to stone formation. The occurrence of diet-induced metabolic acidosis activates kidney compensatory mechanisms to correct the acid-base balance. Removal of non-metallised anions or excretion of ammonium ions can then occur. This decreases urine pH, causing changes in its composition, hypocitraturia, hypercalciuria, and nitrogen and phosphorus loss. This predisposes to the formation of urinary stones [27]. Research by Kim et al. [28] showed that higher glucose levels and HOMA-IR index characterised men with nephrolithiasis. Such changes were not observed in the female population. Factors that predispose to insulin resistance are conditions characteristic of metabolic acidosis, such as insulin resistance in skeletal muscles and increased secretion of glucocorticosteroids and cortisol. Studies suggest that even minor deviations in pH towards metabolic acidosis reduce the sensitivity of tissues to insulin [27]. It can therefore be assumed that among patients with IR, an increased tendency to develop urolithiasis can be expected and vice versa, due to similar mechanisms causing these diseases, such as the presence of systemic metabolic acidosis, MS features, and the presence of inflammatory mediators such as interleukin 6 (IL-6) or tumor necrosis alpha (TNFα).
5. Diet—Insulin Resistance and Acidosis
Patients with IR usually do not achieve the desired dieting therapy results after using a traditional low-calorie diet. In this case, it is necessary to introduce modifications to improve carbohydrate metabolism and reduce tissue resistance to insulin. The selection of the right amount and type of carbohydrates is crucial to lowering excess insulin production, which affects lipid metabolism processes in cells [29].
In countries with highly processed food consumption, urolithiasis is more common in the population, both among adults and children [19]. Foods rich in animal protein increase the production of metabolic acid compounds in the body, such as hydrogen chloride or bisulfate, which may affect the body’s acid-base balance [30][31][32][33][34]. When these foods are consumed, the acids are buffered by the lung excretion of CO2 and the production of sodium salts. These salts are then excreted through the kidneys, mainly with ammonium. Bicarbonate resulting from buffering is returned to the plasma as a substitute for bicarbonate used to soften the acids. When the production of acidic compounds exceeds their excretion via the lungs and kidneys, the amount of bicarbonate in the plasma decreases and thus the blood pH decreases [34]. This mechanism is presented in Figure 2. Studies suggest that treatment of metabolic acidosis with bicarbonate increases insulin sensitivity; therefore, to correct the acid-base balance, it is recommended to significantly increase the amount of alkaline-forming vegetables and fruits in the diet [33][35]. It has been proven that people on a plant-based diet have substantially lower HOMA-IR values and blood glucose levels. It suggests that a diet rich in plant products and limiting animal foods may significantly affect the development of IR [36].
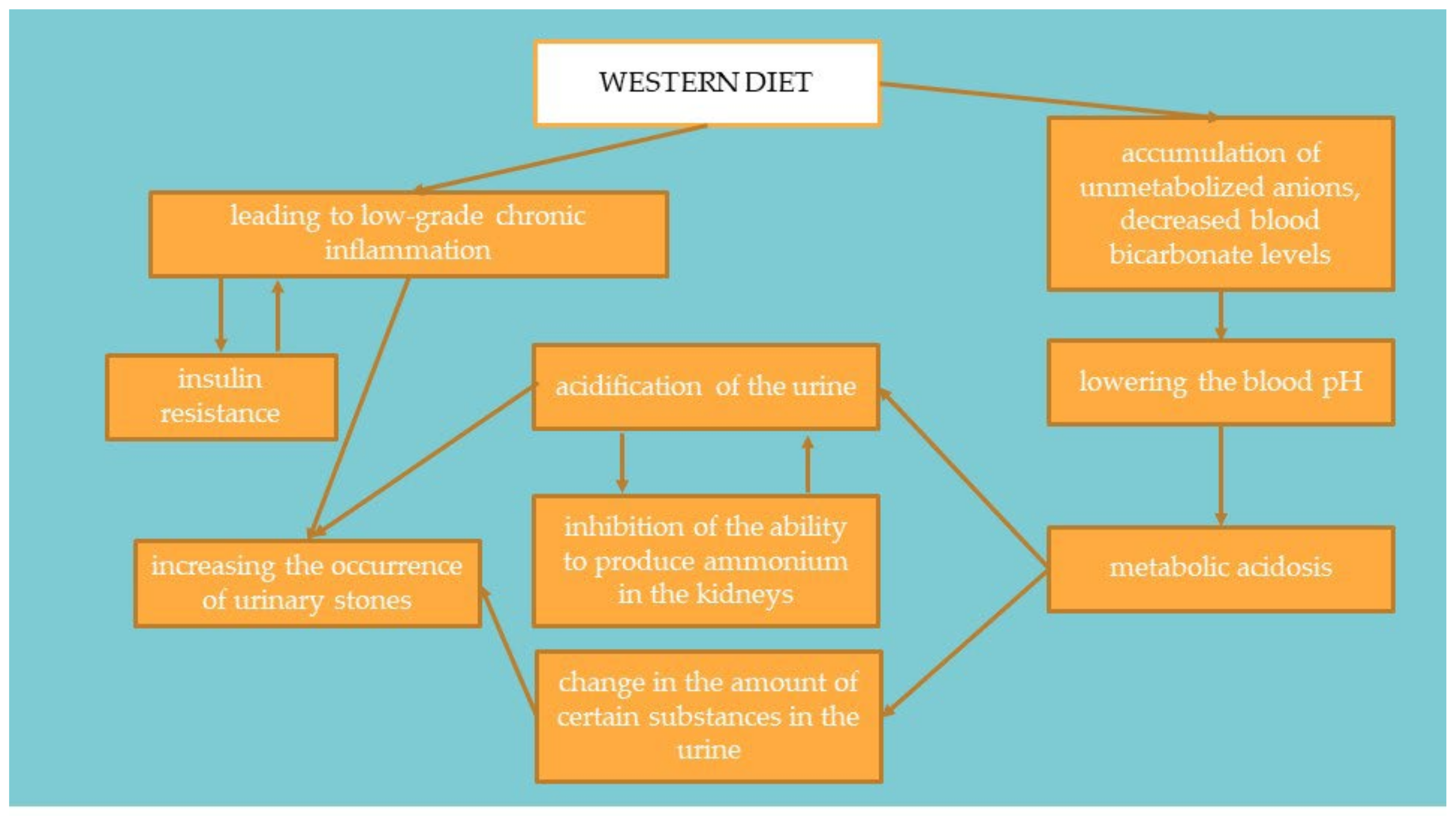
Figure 2. Linking the Western diet with insulin resistance and the occurrence of urolithiasis.
5.1. Carbohydrates
Scientific reports regarding the recommendations for the number of carbohydrates in the diet seem contradictory. They suggest that both a high-carbohydrate diet and a low-carbohydrate diet can benefit patients. Therefore, the number of carbohydrates in dietary therapy should be individually adjusted for each patient based on its effects. Too low an amount of carbohydrates can adversely affect the brain and initiate ketone bodies’ production; too much causes excessive insulin secretion and, consequently, no diet effects. Regardless of the chosen dietary direction, most carbohydrates consumed by the patient should be high-fibre vegetables and then grain products with a low glycemic index. The remaining amount should be filled with fruit and a low index. Berries are especially recommended, such as strawberries, raspberries, and blueberries. It should be emphasised to significantly reduce alcohol consumption, increasing blood glucose [37][38][39][40]. In addition, attention should be paid to the excessive use of sweeteners, which can significantly increase carbohydrate-induced insulin secretion [41].
5.2. Proteins
If it seems justified to reduce the number of carbohydrates in the patient, then the amount of protein should be increased (about 15–20%). However, it should be remembered that too much animal protein causes acidification. Therefore, an appropriate amount of alkaline-forming vegetables and fruits should be selected to reduce the possibility of acidification of the organism [33][35]. The diet’s primary protein sources should be lean meat, unsweetened dairy products without additives (semi-fat products are recommended), legumes, and fatty marine fish containing large amounts of Omega-3 fatty acids. In lipid disorders, it is necessary to reduce red meat significantly. The addition of products including protein to meals with carbohydrates improves the body’s sensitivity to insulin; therefore, it is worth combining dishes, e.g., fruit and kefir cocktails or as additives sauces based on cream or yoghurt for carbohydrate dishes [42][43].
5.3. Fats
Fats delay gastric emptying and absorption of nutrients, including glucose. Thanks to this, they prolong their metabolism. However, it should be pointed out that IR is usually accompanied by overweight or obesity, so the quality of fat taken by the patient is extremely important. Scientific reports indicate that the composition of fatty acids significantly influences IO’s development due to their role in the correct arrangement of cell membrane lipids. Maintaining an adequate omega-three and omega-six fatty acids ratio in the diet is recommended. Cold-pressed unrefined oils are best suited, for example, rapeseed oil or olive oil. Researchers should avoid products rich in saturated fatty acids, such as fatty meat, lard, and palm oil [44][45].
6. Tips for Laying Diets
To properly balance the patient’s diet, follow the rules usually used during composing diets for people with diabetes. It can be included, among others:
-
eliminating juices and sweetened drinks, replacing them with water and tea (herbal, green, red)
-
avoiding fruit in processed form, for example, juices, nectars, mousses, jams, because the sugar they contain is absorbed much faster than in the case of unprocessed fruits
-
properly composed meals—combine products with a higher amount of carbohydrates with products containing fat and protein—this will slow down the absorption of glucose into the blood
-
proper planning of meal times—eating regularly and at regular intervals.
Moreover, it is worth paying attention to the type of food when shopping. Common mistakes made by patients include:
-
choosing highly processed bread, e.g., rice cakes or crispbread, buying bread containing caramel, malt, or honey
-
use of products containing large amounts of salt and preservatives, e.g., broth cubes, spice mixtures, flavour enhancers, Maggi
-
buying light products with low-fat content is often offset by an additional portion of sugar in the product.
These errors often result from the lack of proper nutrition education, which should occur during the first visit to the dietitian.
7. Physical Activity
Physical activity is a critical element of therapy for insulin resistance. During increased physical activity, when the amount of oxygen supplied to the muscles is too low, the cells begin to conduct anaerobic glucose metabolism. This process results in a decrease in ATP in the cell and a simultaneous increase in 5’AMP-activated protein kinase (AMPK), which regulates the body’s homeostasis. Consequently, there is an increase in GLUT 4 (Glucose transporter type 4) in the cell membrane and increased glucose uptake by cells. Moreover, AMPK causes (a) inhibition of cholesterol, (b) TAG synthesis, (c) oxidation of fatty acids in the liver and muscles, and (d) inhibition of lipolysis and (5) lipogenesis in adipocytes. It has been proven that the risk of IR among active people is lower by 33–50% compared to people who do not participate in sports [46][47][48].
This entry is adapted from the peer-reviewed paper 10.3390/ijerph19127160
References
- Meldrum, D.R.; Morris, M.A.; Gambone, J.C. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Fertil. Steril. 2017, 107, 833–839.
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298.
- Gupta, A.; Gupta, V. Metabolic syndrome: What are the risks for humans? BioSci. Trends. 2010, 4, 204–212.
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97.
- Muoio, D.M.; Newgard, C.B. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 193–205.
- Taubes, G. Insulin resistance. Prosperity’s plague. Science 2009, 325, 256–260.
- Landsberg, L. Role of the sympathetic adrenal system in the pathogenesis of the insulin resistance syndrome. Ann. N. Y. Acad. Sci. 1999, 18, 84–90.
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12.
- Haus, J.M.; Solomon, T.P.; Marchetti, C.M.; Edmison, J.M.; González, F.; Kirwan, J.P. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J. Clin. Endocrinol. Metab. 2010, 95, 323–327.
- Dandona, P.; Aljada, A.; Dhindsa, S.; Garg, R. Insulin as an anti-in- flammatory and antiatherosclerotic hormone. Clin. Cornerstone 2003, 4, 13–20.
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801.
- Wesołowski, P.; Wańkowicz, Z. Insulinooporność—Metody rozpoznawania i następstwa kliniczne. Nefrol. Dial. Pol. 2011, 15, 243–246.
- Shimobayashi, M.; Albert, V.; Woelnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538–1550.
- Ovalle, F.; Azziz, R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil. Steril. 2002, 77, 1095–1105.
- Hardy, O.T.; Czech, M.P.; Corvera, S. What causes the insulin resistance underlying obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 81–87.
- Gierach, M.; Gierach, J.; Junik, R. Insulin resistance and thyroid disorders. Endokrynol. Pol. 2014, 65, 70–76.
- Lopez, M.; Hoppe, B. History, epidemiology and regional diversities of urolithiasis. Pediatr. Nephrol. 2010, 25, 49–59.
- Fisang, C.; Anding, R.; Müller, S.; Latz, S.; Laube, N. Urolithiasis—An interdisciplinary diagnostic, therapeutic and secondary preventive challenge. Dtsch. Arztebl. Int. 2015, 112, 83–91.
- Khan, A. Prevalence, pathophysiological mechanisms and factors affecting urolithiasis. Int. Urol. Nephrol. 2018, 50, 799–806.
- Wagner, C.A.; Mohebbi, N. Urinary pH and stone formation. J. Nephrol. 2010, 23, 165–169.
- Ando, R.; Suzuki, S.; Nagaya, T.; Yamada, T.; Okada, A.; Yasui, T.; Tozawa, K.; Tokudome, S.; Kohri, K. Impact of insulin resistance, insulin and adiponectin on kidney stones in the Japanese population. Int. J. Urol. 2011, 18, 131–138.
- Long, X.; Ye, Y.; Zhang, L.; Liu, P.; Yu, W.; Wei, F.; Ren, X.; Yu, J. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways. Int. J. Oncol. 2016, 48, 5–12.
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543–1588.
- Suen, J.L.; Liu, C.C.; Lin, Y.S.; Tsai, Y.F.; Juo, S.H.; Chou, Y.H. Urinary chemokines/cytokines are elevated in patients with urolithiasis. Urol. Res. 2010, 38, 81–87.
- Wang, J.; Wang, W.; Guo, W.; Ma, Y.; Ji, T.; Zhang, B. Clinical importance of chemokines and inflammatory cytokines for patient care following percutaneous nephrolithotripsy. Exp. Ther. Med. 2018, 15, 2189–2195.
- Spatola, L.; Ferraro, P.M.; Gambaro, G.; Badalamenti, S.; Dauriz, M. Metabolic syndrome and uric acid nephrolithiasis: Insulin resistance in focus. Metabolism 2018, 83, 225–233.
- Adeva, M.M.; Souto, G. Diet-induced metabolic acidosis. Clin. Nutr. 2011, 30, 416–421.
- Kim, S.; Chang, Y.; Jung, H.S.; Hyun, Y.Y.; Lee, K.B.; Joo, K.J.; Park, H.J.; Cho, Y.S.; Ko, H.; Sung, E.; et al. Glycemic Status, Insulin Resistance, and the Risk of Nephrolithiasis: A Cohort Study. Am. J. Kidney Dis. 2020, 76, 658–668.
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495.
- Frassetto, L.A.; Todd, K.M.; Morris, R.C., Jr.; Sebastian, A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am. J. Clin. Nutr. 1998, 68, 576–583.
- Remer, T. Influence of nutrition on acid-base balance—Metabolic aspects. Eur. J. Nutr. 2001, 40, 214–220.
- Della Guardia, L.; Roggi, C.; Cena, H. Diet-induced acidosis and alkali supplementation. Int. J. Food Sci. Nutr. 2016, 67, 754–761.
- Della Guardia, L.; Thomas, M.A.; Cena, H. Insulin Sensitivity and Glucose Homeostasis Can Be Influenced by Metabolic Acid Load. Nutrients 2018, 10, 618.
- Williams, R.S.; Kozan, P.; Samocha-Bonet, D. The role of dietary acid load and mild metabolic acidosis in insulin resistance in humans. Biochimie 2016, 124, 171–177.
- Bellasi, A.; Di Micco, L.; Santoro, D.; Marzocco, S.; De Simone, E.; Cozzolino, M.; Di Lullo, L.; Guastaferro, P.; Di Iorio, B. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 2016, 17, 158.
- Barnard, N.D.; Scialli, A.R.; Turner-McGrievy, G.; Lanou, A.J.; Glass, J. The effects of a low-fat, plant-based dietary Intervention on body weight, metabolism, and insulin sensitivity. Am. J. Med. 2005, 118, 991–997.
- Due, A.; Larsen, T.M.; Hermansen, K.; Stender, S.; Holst, J.J.; Toubro, S.; Martinussen, T.; Astrup, A. Comparison of the effects on insulin resistance and glucose tolerance of 6-mo high-monounsaturated-fat, low-fat, and control diets. Am. J. Clin. Nutr. 2008, 87, 855–862.
- Kahleova, H.; Dort, S.; Holubkov, R.; Barnard, N.D. A Plant-Based High-Carbohydrate, Low-Fat Diet in Overweight Individuals in a 16-Week Randomised Clinical Trial: The Role of Carbohydrates. Nutrients 2018, 10, 1302.
- Barazzoni, R.; Deutz, N.E.P.; Biolo, G.; Bischoff, S.; Boirie, Y.; Cederholm, T.; Cuerda, C.; Delzenne, N.; Leon Sanz, M.; Ljungqvist, O.; et al. Carbohydrates and insulin resistance in clinical nutrition: Recommendations from the ESPEN expert group. Clin. Nutr. 2017, 36, 355–363.
- O’Neill, B.J. Effect of low-carbohydrate diets on cardiometabolic risk, insulin resistance, and metabolic syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 301–307.
- Kolb, H.; Kempf, K.; Röhling, M.; Martin, S. Insulin: Too much of a good thing is bad. BMC Med. 2020, 18, 224.
- Yu, Z.; Nan, F.; Wang, L.Y.; Jiang, H.; Chen, W.; Jiang, Y. Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes: A systematic Rev.iew and meta-analysis of randomised controlled trials. Clin. Nutr. 2020, 39, 1724–1734.
- Ouellet, V.; Marois, J.; Weisnagel, S.J.; Jacques, H. Dietary Cod Protein Improves Insulin Sensitivity in Insulin-Resistant Men and Women. Diabetes Care 2007, 30, 2816–2821.
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39.
- Frost, G.S.; Brynes, A.E.; Dhillo, W.S.; Bloom, S.R.; McBurney, M.I. The effects of fiber enrichment of pasta and fat content on gastric emptying, GLP-1, glucose, and insulin responses to a meal. Eur. J. Clin. Nutr. 2003, 57, 293–298.
- Riek, U.; Scholz, R.; Konarev, P.; Rufer, A.; Suter, M.; Nazabal, A.; Ringler, P.; Chami, M.; Müller, S.A.; Neumann, D.; et al. Structural properties of AMP-activated protein kinase: Dimerisation, molecular shape, and changes upon ligand binding. J. Biol. Chem. 2008, 283, 18331–18343.
- LaMonte, M.J.; Eisenman, P.A.; Adams, T.D.; Shultz, B.B.; Ainsworth, B.E.; Yanowitz, F.G. Cardiorespiratory fitness and coronary heart disease risk factors: The LDS. Hospital Fitness Institute cohort. Circulation 2000, 102, 1623–1628.
- Balkau, B.; Vernay, M.; Mhamdi, L.; Novak, M.; Arondel, D.; Vol, S.; Tichet, J.; Eschwège, E. The incidence and persistence of the NCEP (National Cholesterol Education Program) metabolic syndrome. The French DESIR study. Diabetes Metab. 2003, 29, 526–532.
This entry is offline, you can click here to edit this entry!
