Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
The therapeutic landscape in patients with advanced non-small-cell lung cancer harboring oncogenic biomarkers has radically changed with the development of targeted therapies. Although lung cancers are known to frequently metastasize to the brain, oncogene-driven non-small-cell lung cancer patients show a higher incidence of both brain metastases at baseline and a further risk of central nervous system progression/relapse.
- NSCLC
- oncogenic biomarkers
- brain metastases
1. Introduction
Lung cancer is one of the most commonly diagnosed cancers (11.6% of all new tumors) [1]. Despite a decline in the death rate in recent years, lung cancer is still the leading cause of cancer deaths [2]. Non-small-cell lung cancer (NSCLC) accounts for 85% of lung cancers, with 60–70% of patients presenting at either stage III B or stage IV of the disease [3].
Brain metastases (BMs) are a common complication in a wide range of cancers, but they are particularly common among NSCLC patients. Indeed, the incidence rate of BMs at diagnosis is 10–20% and up to 40% during the course of the disease [4,5,6].
In recent times, the lifetime incidence of the central nervous system (CNS) metastases in NSCLC has increased as a result of both improved neuro-imaging techniques by the use of magnetic resonance imaging and furthermore of an increase in patient survival because of better systemic control of extracranial disease.
In patients with metastatic lung cancer, BMs are associated with inferior health-related quality of life (QoL) and poor prognosis (life expectancy ranging between 3 and 13 months) [7].
Historically, CNS has been considered a pharmacological sanctuary because of the physical and chemical characteristics of the blood–brain barrier (BBB), a diffusion barrier essential for CNS function. The continuous tight junctions that join the endothelial cells in the brain capillaries limit the influx of circulating factors from blood to brain [8].
Local treatments have traditionally been the cornerstone of BMs management. Surgical resection (SR), stereotactic radiosurgery (SRS), and whole-brain radiation therapy (WBRT) have been the primary treatment modalities.
Brain irradiation can significantly impair BBB integrity.
WBRT is associated with a significant rate of neurological toxicities, and acute, early-delayed, and late effects. Unlike acute neurological symptoms, which are usually reversible, long-term effects appear several months or years later and are generally irreversible [9,10,11]. Partial brain RT may also have late effects on cognitive function, although the risk is lower than with WBRT. Figure 1
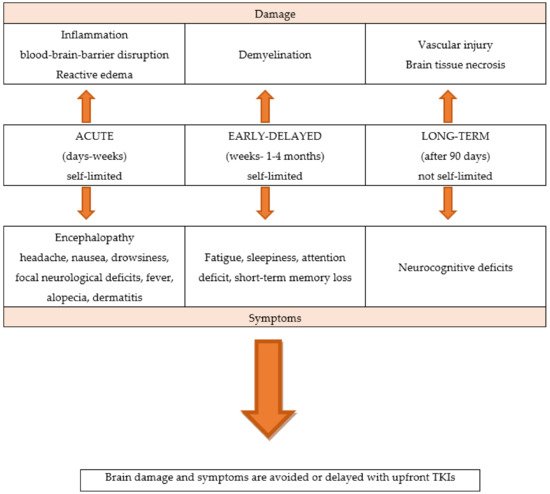
Figure 1. The neurological toxicities of brain radiotherapy.
The selection of local treatment is based on the number of BMs, the size or location of BMs, the symptoms of encephalic disease, and the status of extracranial metastases.
For patients with symptomatic or a limited number of BMs, who have a controlled primary disease or are suitable for radical treatment, local therapy with a neurosurgical resection or with SRS is recommended. Instead, WBRT is considered for pluri-metastatic CNS disease [12].
SR is often the standard of care (SoC) for solitary or symptomatic brain metastasis, and it can provide immediate and effective relief from symptomatic mass effects [13,14]. The combination of the neurosurgical resection of solitary brain metastasis and postoperative RT favored the combination treatment. A phase III randomized control trial compared post-operative SRS to the surgical cavity with WBRT in patients who underwent resection of a single brain metastasis and demonstrated a lower probability of deterioration in cognitive function and no difference in overall survival (OS) [15]; thus, adjuvant SRS to the surgical resection bed should be considered the preferred local therapy.
A systematic review did not show a significant outcome difference between SR and SRS in patients with single brain metastasis [16].
SRS alone has become the SoC for patients with a good performance status, who cannot undergo resection and/or have a limited number of BMs (1–4 BMs) [17].
SRS allows many precisely focused radiation beams, improving healthy tissue preservation and less cognitive decline.
In past decades, WBRT was the most widely used local treatment for the management of patients with multiple BMs. The radiation schedules include a classical dose of 30 gray (Gy) in 10 fractions or a short course of 20 Gy in 5 fractions, showing similar efficacy [18].
In the last few years, the role of WBRT is declining because of the potential cognitive deficits and the limited clinical benefit compared with best supportive care [19,20].
Therefore, because of its high risk of cognitive function deterioration and unproven survival benefit, the WBRT could be an upfront treatment option for patients with symptomatic BMs; alternatively, a wait-and-see approach could be adopted.
Cytotoxic chemotherapy (CT) plays a limited role in controlling BMs because of the drugs’ inability to cross the BBB and penetrate the CNS [21].
Platinum-based regimens have been the most commonly used therapy for metastatic NSCLC. These CT regimens demonstrated low systemic response rate (RR) in a few brain RT-naïve patients [22].
Thus, upfront CT could represent the better option then WBRT in NSCLC patients with multiple asymptomatic BMs, who are not eligible for SRS. Therefore, WBRT could be reserved for symptomatic patients with good performance status or for intracranial non-responders.
In recent years, both single-agent immune checkpoint inhibitors (ICIs) and a combination of CT plus ICIs have shown better efficacy than platinum-based regimens in NSCLC patients, who do not harbor a driver oncogene alteration in randomized phase III clinical trials. A future critical challenge is knowing how to identify NSCLC patients with CNS disease who benefit from ICIs and the potential of combining radiation with ICIs.
Recently, routine molecular testing has become the SoC for determining the optimal treatment of newly diagnosed advanced or metastatic NSCLC patients. In particular, a range of predictive and prognostic biomarkers have been identified in adenocarcinoma: epidermal growth factor receptor (EGFR) mutations, anaplastic lymphoma kinase (ALK) rearrangements, c-ros oncogene 1 (ROS1) rearrangements, v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) mutations, Kirsten rat sarcoma viral oncogene homologue (KRAS) mutations, neurotrophic receptor tyrosine kinase (NTRK) 1/2/3 rearrangements, rearranged during transfection (RET) rearrangements, N-methyl-N′-nitroso-guanidine human osteosarcoma transforming gene (MET) exon14 skipping mutations, and activating human epidermal growth factor receptor 2 (HER2) mutations [23,24,25]. Figure 2
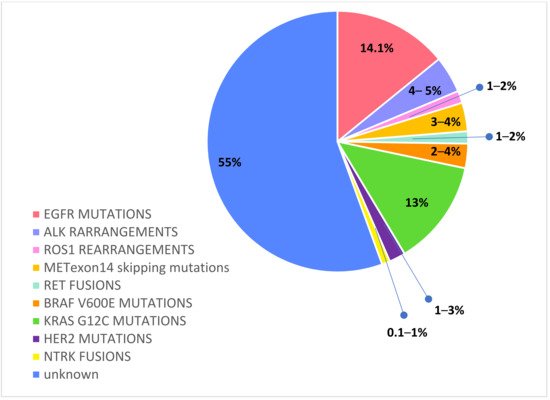
Figure 2. Druggable molecular alterations with tailored treatments in oncogene-addicted NSCLC.
According to previous reports, driver oncogenic alterations, such as EGFR, ALK, ROS1, RET, NTRK, and HER2, have a higher frequency in never smokers, younger age, females, and Asian NSCLC patients, with a tendency to early metastasis and to brain dissemination [26].
The sequencing of the human genome has permitted one to characterize different molecular subgroups of lung cancer patients, who are grouped under the definition of oncogene-addicted NSCLC.
The identification of an ever-increasing number of potentially druggable molecular alterations has led to the development of tailored treatments, which are tyrosine kinase inhibitors (TKIs), with remarkable results in terms of intracranial disease control and OS. Multiple randomized phase III studies demonstrated the superiority of first-line targeted therapies over platinum-based CT for oncogene-addicted NSCLC patients.
Compared to non-oncogenic-driven NSCLC, the prognosis of mutated patients has continuously improved. NSCLC patients with oncogenic-driven mutations are more likely to develop BMs because of the better control of extracranial disease and prolonged survival.
Given the significant CNS activity of novel targeted agents, there is growing evidence that upfront treatment with tailored systemic therapies can sufficiently control BMs.
There are several reasons why local brain irradiation can be deferred in favor of first-line next-generation TKIs:
-
The newer generation targeted systemic therapies have demonstrated far greater CNS penetration than CT or older targeted agents; the newer molecular targeted agents are liposoluble compounds with low molecular weight and can cross the BBB; furthermore, they have the ability to penetrate cerebrospinal fluid (CSF).
-
Oncogene-addicted NSCLC patients are living several years rather than only a few months, allowing for more time for BMs to develop, as well as for adverse effects from prior RT to manifest.
-
These factors lead to a treatment strategy shift, privileging brain penetrant TKIs systemic therapies over local treatments, maintaining patient QoL by minimizing the RT-related consequences.
In the era of targeted therapies, the management of BMs is a challenging issue. It remains unclear whether it is reasonable enough to defer RT until the intracranial progression is noted in patients on TKIs; therefore, the question of how and when to perform brain RT remains open.
Studies on the clinical efficacy of RT combined with TKIs for patients with CNS metastases are very limited and largely retrospective; most adopt only WBRT, and many prospective trials recruit relatively small numbers of patients without considering oncogenic mutational status [27,28,29]. Furthermore, data for patients with molecular driver alterations other than those EGFR are even more scarce, and the efficacy of combined RT with these agents is only anecdotal [30].
Perspective trials appropriately designed to assess the optimal timing of brain RT are necessary.
2. Egfr Mutations
Activated EGFR mutations, predominantly exon 19 deletions and exon 21 L858R mutations, occur in approximately 14.1% of Caucasian NSCLC [31].
Among EGFR mutated NSCLC patients, BMs have an increased frequency, considering baseline incidence ranging from 23% to 32% [32,33,34,35] and a further risk of intracranial progression of about 15–20% during first-generation TKIs treatment [36,37].
These data reflect a pharmacokinetic failure of the first- and second- generation EGFR TKIs to penetrate the brain. Though erlotinib, gefitinib, and afatinib have intracranial activity, these agents have a limited BBB penetration, and they are detected in CFS only at a low concentration, in the 1 to 5 percent range of what is observed in the serum [38,39,40,41].
By contrast, third-generation irreversible TKI osimertinib achieves a greater intracerebral concentration and has shown high intracranial response rates, even against leptomeningeal carcinomatosis [42,43,44].
Osimertinib was first approved in the second-line setting in patients that developed a T790M mutation after failure of a first-generation TKI.
Pooled data from two phase II trials—AURA extension and AURA2—in 50 T790M-positive advanced NSCLC patients with BMs progressed to prior EGFR TKI have demonstrated the significant intracranial activity of osimertinib; CNS objective response rate (ORR) and disease control rate (DCR) were 54% and 92%, respectively, and CNS response was observed regardless of prior brain irradiation [45].
In the randomized phase III AURA 3 trial osimertinib demonstrated significantly greater progression-free survival (PFS) than platinum-based doublet-CT in patients with EGFR T790M advanced NSCLC and progression on prior EGFR-TKI treatment. Among 116 patients with BMs (measurable or not), PFS was longer with osimertinib compared to CT (11.7 vs. 5.6 months, HR 0.32; and 95% CI: 0.15–0.69) and cumulative incidence of CNS progression at 6 months was lower with osimertinib compared to CT (11.5% vs. 28.2%) [46].
Subsequently, osimertinib was approved in the first-line setting on the basis of the randomized phase III FLAURA trial, which evaluated the efficacy of upfront osimertinib versus a SoC EGFR TKI (erlotinib or gefitinib) in treatment-naïve EGFR mutant (exon 19 del or L858R) advanced NSCLC patients [47].
The CNS activity of osimertinib was confirmed in a subset analysis of the randomized phase III FLAURA trial, which evaluated the efficacy of upfront osimertinib versus a SoC EGFR TKI (erlotinib or gefitinib) in treatment-naïve EGFR mutant (exon 19 del or L858R) advanced NSCLC patients. In the preplanned, exploratory analysis (CNS analysis set, N = 128), osimertinib reported that improved CNS RR (66% vs. 43%) and median CNS PFS among patients with measurable and/or non-measurable CNS lesions was longer (not reached vs. 13.9 months, HR 0.48, 95% CI: 0.26–0.86, and p = 0.04). Furthermore, osimertinib reduced the risk of CNS progression in the overall study population (6% vs. 15%), regardless of the presence or absence of known or treated CNS metastases at baseline. Among patients with BMs evaluable for response (N = 41), osimertinib improved the CNS RR (91% vs. 68%) [48].
Data from this analysis show that osimertinib reveal encouraging activity against CNS involvement, with a greater intracranial response and clinical benefit both in preventing or delaying BMs.
Randomized trials comparing upfront osimertinib with brain irradiation are lacking. Although retrospective data indicate that the deferral of RT may be associated with worse outcomes compared with early RT [49,50,51,52,53], those studies were all conducted with earlier-generation EGFR TKIs, which have less intracranial activity than osimertinib.
In sum, the available data suggest that osimertinib demonstrates the greatest CNS activity and prevention of CNS progression, making it the preferred initial treatment option for EGFR-mutated NSCLC with BMs, deferring brain RT and its neurocognitive defects in case of intracranial progression.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23126477
This entry is offline, you can click here to edit this entry!
