Mitochondria, as the main site of cellular energy metabolism and the generation of oxygen free radicals, are the key switch for mitochondria-mediated endogenous apoptosis. Ca2+ is not only an important messenger for cell proliferation, but it is also an indispensable signal for cell death. Ca2+ participates in and plays a crucial role in the energy metabolism, physiology, and pathology of mitochondria. Mitochondria control the uptake and release of Ca2+ through channels/transporters, such as the mitochondrial calcium uniporter (MCU), and influence the concentration of Ca2+ in both mitochondria and cytoplasm, thereby regulating cellular Ca2+ homeostasis. Mitochondrial Ca2+ transport-related processes are involved in important biological processes of tumor cells including proliferation, metabolism, and apoptosis.
- mitochondrial calcium
- calcium homeostasis
1. Introduction
2. Regulation of Mitochondrial Ca2+
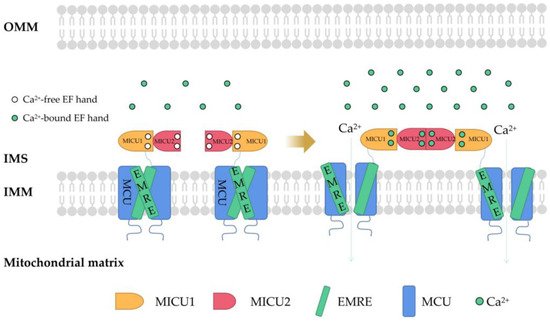
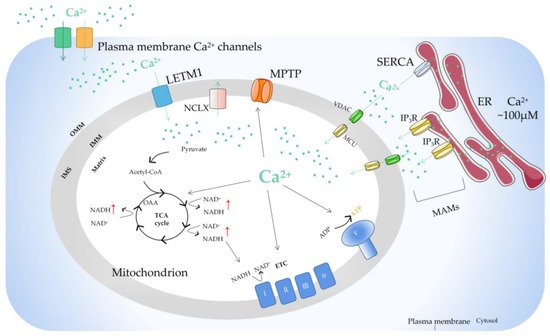
This entry is adapted from the peer-reviewed paper 10.3390/ijms23126667
References
- Nunnari, J.; Suomalainen, A. Mitochondria: in sickness and in health. Chem Commun (Camb). 2012, 148, 1145-1159.
- Bravo-Sagua, R.; Parra, V.; López-Crisosto, C.; Díaz, P.; Quest, AF.; Lavandero, S. Calcium Transport and Signaling in Mitochondria. Compr Physiol. 2017, 7, 623-634.
- Patergnani, S.; Danese, A.; Bouhamida, E.; Aguiari, G.; Previati, M.; Pinton, P.; Giorgi, C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int J Mol Sci. 2020, 21, 8323.
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018, 19, 713-730.
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Intracellular calcium homeostasis and signaling. Met Ions Life Sci. 2013, 12, 119-168.
- Carafoli, E.; Krebs, J. Why Calcium? How Calcium Became the Best Communicator. JBC. 2016, 40, 20849-20857.
- Zavodnik, IB. Mitochondria, calcium homeostasis and calcium signaling. Biomed Khim. 2016, 62, 311-317.
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003, 4, 552-565.
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, MR.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018, 69, 62-72.
- Magalhães, PJ.; Rizzuto, R. Mitochondria and calcium homeostasis: a tale of three luminescent proteins. Luminescence. 2001, 16, 67-71.
- Godoy, JA.; Rios, JA.; Picón-Pagès, P.; Herrera-Fernández, V.; Swaby, B.; Crepin, G.; Vicente, R.; Fernández-Fernández, JM.; Muñoz, FJ. Mitostasis, Calcium and Free Radicals in Health, Aging and Neurodegeneration. Biomolecules. 2021, 11, 1012.
- Robb-Gaspers, L.; Burnett, P.; Rutter, G.; Denton, R.; Rizzuto, R.; Thomas, A. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 1998, 17, 4987-5000.
- Uhlén, P.; Fritz, N. Biochemistry of calcium oscillations. Biochem Biophys Res Commun. 2010, 396, 28-32.
- Rizzuto, R.; Brini, M.; Murgia, M.; Pozzan, T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993, 262, 744-747.
- Hajnóczky, G.; Robb-Gaspers, LD.; Seitz, MB.; Thomas, AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995, 82, 415-424.
- Sheu, SS.; Jou, MJ. Mitochondrial free Ca2+ concentration in living cells. J Bioenerg Biomembr. 1994, 26, 487-493.
- Jouaville, LS.; Pinton, P.; Bastianutto, C.; Rutter, GA.; Rizzuto, R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999, 96, 13807-13812.
- Berridge, MJ.; Bootman, MD.; Roderick, HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003, 4, 517-529.
- Paudel, S.; Sindelar, R.; Saha, M. Calcium Signaling in Vertebrate Development and Its Role in Disease. Int J Mol Sci. 2018, 19, 3390.
- Pézier, A.; Acquistapace, A.; Renou, M.; Rospars, JP.; Lucas, P. Ca2+ stabilizes the membrane potential of moth olfactory receptor neurons at rest and is essential for their fast repolarization Chem Senses. 2007, 32, 305-317.
- Pendin, D.; Greotti, E.; Filadi, R.; Pozzan, T. Spying on organelle Ca2+ in living cells: the mitochondrial point of view. J Endocrinol Invest. 2015, 38, 39-45.
- Yamamoto, T. The Molecular Mechanisms of Mitochondrial Calcium Uptake by Calcium Uniporter. Yakugaku Zasshi J. Pharm. Jpn. 2021, 141, 491–499.
- Stefani, DD.; Raffaello, A.; Teardo, E.; Szabò, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011, 476, 336-340.
- Belosludtsev, KN.; Dubinin, MV.; Belosludtseva, NV.; Mironova, GD. Mitochondrial Ca2+ Transport: Mechanisms, Molecular Structures, and Role in Cells. Biochemistry (Mosc). 2019, 84, 593-607.
- Baughman, JM.; Perocchi, F.; Girgis, HS.; Plovanich, M.; Belcher-Timme, CA.; Sancak, Y.; Bao, XR.; Strittmatter, L.; Goldberger, O.; Bogorad, RL.; Koteliansky, V.; Mootha, VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011, 476, 341-345.
- Pan, X.; Liu, J.; Nguyen, T.; Liu, C.; Sun, J.; Teng, Y.; Fergusson, MM.; Rovira, II.; Allen, M.; Springer, DA.; Aponte, AM.; Gucek, M.; Balaban, RS.; Murphy, E.; Finkel, T. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013, 15, 1464-1472.
- Garbincius, J.; Elrod, J. Mitochondrial calcium exchange in physiology and disease. Physiol Rev. 2022, 102, 893-992.
- Chen, L.; Sun, Q.; Zhou, D.; Song, W.; Yang, Q.; Ju, B.; Zhang, L.; Xie, H.; Zhou, L.; Hu, Z.; Yao, H.; Zheng, S.; Wang, W. HINT2 triggers mitochondrial Ca2+ influx by regulating the mitochondrial Ca2+ uniporter (MCU) complex and enhances gemcitabine apoptotic effect in pancreatic cancer. Cancer Lett. 2017, 411, 106–116.
- Curry, MC.; Peters, AA.; Kenny, PA.; Roberts-Thomson, SJ.; Monteith, GR. Mitochondrial calcium uniporter silencing potentiates caspase-independent cell death in MDA-MB-231 breast cancer cells. Biochemical and Biophysical Research Communications. 2013, 434, 695-700.
- Sancak, Y.; Markhard, A.; Kitami, T.; Kovacs-Bogdan, E.; Kamer, K.; Udeshi, N.; et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013, 342, 1379-1382.
- Wang, W.; Xie, Q.; Zhou, X.; Yao, J.; Zhu, X.; Huang, P.; Zhang, L.; Wei, J.; Xie, H.; Zhou, L.; Zheng, S. Mitofusin-2 triggers mitochondria Ca2+ influx from the endoplasmic reticulum to induce apoptosis in hepatocellular carcinoma cells. Cancer Lett. 2015, 358, 47–58.
- Zhuo, W.; Zhou, H.; Guo, R.; Yi, J.; Zhang, L.; Yu, L.; et al. Structure of intact human MCU supercomplex with the auxiliary MICU subunits. Protein & Cell. 2020, 12, 220-229.
- Fan, M.; Zhang, J.; Tsai, C.; Benjamin, J.; Rodriguez, M.; Xu, Y.; Liao, M.; Tsai, M.; Feng, L. Structure and mechanism of the mitochondrial Ca2+ uniporter holocomplex. Nature. 2020, 582, 129-133.
- Tomar, D.; Elrod, JW. Metabolite regulation of the mitochondrial calcium uniporter channel. Cell Calcium. 2020, 92, 102288.
- Gottschalk, B.; Madreiter-Sokolowski, CT.; Graier, WF. Cristae junction as a fundamental switchboard for mitochondrial ion signaling and bioenergetics. Cell Calcium. 2022, 101, 102517.
- Gottschalk, B.; Klec, C.; Leitinger, G.; Bernhart, E.; Rost, R.; Bischof, H.; Madreiter-Sokolowski, CT.; Radulović, S.; Eroglu, E.; Sattler, W.; Waldeck-Weiermair, M.; Malli, R.; Graier, WF. MICU1 controls cristae junction and spatially anchors mitochondrial Ca2+ uniporter complex. Nat Commun. 2019, 10, 3732.
- Chakraborty, PK.; Mustafi, SB.; Xiong, X.; Dwivedi, SKD.; Nesin, V.; Saha, S.; Zhang, M.; Dhanasekaran, D.; Jayaraman, M.; Mannel, R.; Moore, K.; McMeekin, S.; Yang, D.; Zuna, R.; Ding, K.; Tsiokas, L.; Bhattacharya, R.; Mukherjee, P. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat Commun. 2017, 8 14634.
- Trenker, M.; Malli, R.; Fertschai, I.; Levak-Frank, S.; Graier, WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol. 2007, 9, 445-452.
- Madreiter-Sokolowski, CT.; Klec, C.; Parichatikanond, W.; Stryeck, S.; Gottschalk, B.; Pulido, S.; Rost, R.; Eroglu, E.; Hofmann, NA.; Bondarenko, AI.; Madl, T.; Waldeck-Weiermair, M.; Malli, R.; Graier, WF. PRMT1-mediated methylation of MICU1 determines the UCP2/3 dependency of mitochondrial Ca(2+) uptake in immortalized cells. Nat Commun. 2016, 7, 12897.
- Madreiter-Sokolowski, CT.; Győrffy, B.; Klec, C.; Sokolowski, AA.; Rost, R.; Waldeck-Weiermair, M.; Malli, R.; Graier, WF. UCP2 and PRMT1 are key prognostic markers for lung carcinoma patients. Oncotarget. 2017, 8, 80278-80285.
- Jarrold, J.; Davies, CC. PRMTs and Arginine Methylation: Cancer's Best-Kept Secret? Trends Mol Med. 2019, 25, 993-1009.
- Payne, R.; Hoff, H.; Roskowski, A.; Foskett, J. MICU2 restricts spatial crosstalk between InsP3R and MCU channels by regulating threshold and gain of MICU1‐mediated inhibition and activation of MCU. Cell Reports. 2017, 21, 3141–3154.
- Patron, M.; Granatiero, V.; Espino, J.; Rizzuto, R.; De Stefani, D. MICU3 is a tissue‐specific enhancer of mitochondrial calcium uptake. Cell Death and Differentiation. 2019, 26, 179–195.
- Raffaello, A.; De Stefani, D.; Sabbadin, D.; Teardo, E.; Merli, G.; Picard, A.; Checchetto, V.; Moro, S.; Szabò, I.; Rizzuto, R. The mitochondrial calcium uniporter is a multimer that can include a dominant‐negative pore‐forming subunit. The EMBO Journal. 2013, 32, 2362–2376.
- Tomar, D.; Dong, Z.; Shanmughapriya, S.; Koch, D.; Thomas, T.; Hoffman, N.; Timbalia, S.; Goldman, S.; Breves, S.; Corbally, D.; et al. MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics. Cell Rep. 2016, 15, 1673-1685.
- Chaudhuri, D.; Artiga, D. J.; Abiria, S. A.; Clapham, D. E. Mitochondrial calcium uniporter regulator 1 (MCUR1) regulates the calcium threshold for the mitochondrial permeability transition. Proceedings of the National Academy of Sciences of the United States of America. 2016, 113, E1872–E1880.
- Roy, S.; Dey, K.; Hershfinkel, M.; Ohana, E.; Sekler, I. Identification of residues that control Li+ versus Na+ dependent Ca2+ exchange at the transport site of the mitochondrial NCLX. Biochim Biophys Acta Mol Cell Res. 2017, 1864, 997-1008.
- Starkov, AA.; Chinopoulos, C.; Starkova, NN.; Konrad, C.; Kiss, G.; Stepanova, A.; Popov, VN. Divalent cation chelators citrate and EDTA unmask an intrinsic uncoupling pathway in isolated mitochondria. J Bioenerg Biomembr. 2017, 49, 3-11.
- Wilson, JA.; Lau, YS.; Gleeson, JG.; Wilson, JS. The action of MPTP on synaptic transmission is affected by changes in Ca2+ concentrations. Brain Res. 1991, 541, 342-346.
- Kolomiiets', O.; Danylovych, I.; Danylovych, H. H+-Ca2+-exchanger in the myometrium mitochondria: modulation of exogenous and endogenous compounds. Fiziol Zh. 2014, 60, 33-42.
- Gomez-Suaga, P.; Paillusson, S.; Stoica, R.; Noble, W.; Hanger, DP.; Miller, CC. The ER-mitochondria tethering complex VAPB-PTPIP51 regulates autophagy. Curr Biol. 2017, 27, 371-385.
- Giorgi, C.; Bonora, M.; Sorrentino, G.; Missiroli, S.; Poletti, F.; Suski, JM.; Galindo Ramirez, F.; Rizzuto, R.; Di Virgilio, F.; Zito, E.; Pandolfi, PP.; Wieckowski, MR.; Mammano, F.; Del Sal, G.; Pinton, P. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc Natl Acad Sci U S A. 2015, 112, 1779-1784.
- Betz, C.; Stracka, D.; Prescianotto-Baschong, C.; Frieden, M.; Demaurex, N.; Hall, MN. Feature Article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Natl Acad Sci U S A. 2013, 110, 12526-12534.
- Rimessi, A.; Marchi, S.; Patergnani, S.; Pinton, P. H-Ras-driven tumoral maintenance is sustained through caveolin-1-dependent alterations in calcium signaling. Oncogene. 2014, 33, 2329-2340.
- Glitsch, M. Mechano- and pH-sensing convergence on Ca2+-mobilising proteins - A recipe for cancer? Cell Calcium. 2019, 80, 38-45.
- Trapani, V.; Wolf, FI. Dysregulation of Mg2+ homeostasis contributes to acquisition of cancer hallmarks. Cell Calcium. 2019, 83, 102078.
- Santoni, G.; Morelli, MB.; Marinelli, O.; Nabissi, M.; Santoni, M.; Amantini, C. Calcium Signaling and the Regulation of Chemosensitivity in Cancer Cells: Role of the Transient Receptor Potential Channels. Adv Exp Med Biol. 2020, 1131, 505-517.
