This review provides general information on the possible health benefits in
animals and humans of herbal additives, particularly thymol, whose phenolic group is responsible
for the neutralisation of free radicals, and information concerning its detection through body action,
bioavailability and mechanisms in rabbits. Plants containing thymol have been used in traditional
medicine for the treatment of various diseases, such as cardiovascular diseases, cancer and diabetes.
Although a great number of in vitro studies of cardiovascular and cancer diseases are available,
in vivo studies that confirm these findings have not been sufficiently reported. To determine the
beneficial dose, further clinical studies are necessary, with preclinical comprehensive research on
animal models.
- thymol
- biological activity
- health
- human
- animal
1. Introduction
2. Detection of Thymol and Its Metabolites in Humans and Animals
The pharmacodynamic activities of thyme extract or essential oil have been demonstrated in vitro. To confirm the beneficial effect of thymol found in vitro, its absorption, distribution, metabolism and excretion need to be detected in vivo [55]. Little is known about the bioactivity of thymol and its metabolites, as there are only few studies that have analysed thymol in body tissues (Table 1).
| Animal Species |
Applied Form | Detectable Compounds | Samples | References |
|---|---|---|---|---|
| human | thymol/orally | thymol glucuronide thymol sulphate thymohydroquinone sulphate thymol |
urine | [59] |
| human | Bronchipret® TP/orally (equivalent to 1.08 mg thymol) |
thymol sulphate | plasma, urine | [55] |
| thymol glucuronide | urine | |||
| human | thymol/orally | p-cymene-2,5-diol p-cymene-2,3-diol p-cymene-3-ol-8-ene |
urine | [62] |
| human | dried thyme/orally | thymol sulphate caffeic acid sulphate hydroxyphenylpropionic acid sulphate |
plasma | [67] |
| thymol sulphate caffeic acid sulphate hydroxyphenylpropionic acid sulphate thymol glucuronide |
urine | |||
| rabbit | thymol/orally | glucuronic acid ethereal sulphuric acid thymol |
urine | [59] |
| rabbit | thymol/orally | thymol | plasma, small intestinal wall, liver, kidney, spleen, caecum, colon, muscle, faeces | [58] |
| rat | thymol/orally | p-cymene-2,5-diol p-cymene-3,9-diol p-cymene-3,7-diol thymol |
urine | [60] |
| rat | thyme extract/orally | thymol sulphate | plasma | [68] |
| laying hen | thyme extract/orally | p-cymene-2,3-diol thymol |
egg yolk | [61] |
| Japanese quail | thymol/orally | thymol | egg yolk, faeces | [74] |
| broiler chicken | dried Thymi herba/orally | thymol | plasma, small intestine, caecum, liver, muscle | [70] |
| broiler chicken | thyme essential oil/orally | thymol | plasma, liver, kidney, muscle, duodenal wall, gut content | [63] |
| broiler chicken | thyme essential oil/orally | thymol sulphate thymol glucuronide |
plasma, duodenal wall, liver | [76] |
| piglet | essential oil/orally (carvacrol, thymol, eugenol and trans-cinnamaldehyde) |
carvacrol thymol eugenol |
plasma | [64] |
| carvacrol thymol eugenol trans-cinnamaldehyde |
small intestine | |||
| carvacrol thymol eugenol trans-cinnamaldehyde |
bile | |||
| carvacrol thymol eugenol |
urine | |||
| piglet | Biomin® P.E.P 1000 (main compounds thymol and carvacrol)/orally | thymol | plasma, kidney, faeces | [72] |
| piglet | Thymi herba/orally | thymol | plasma | [73] |
| bovine | Phyto-Mast (essential oil of Thymus vulgaris and oregano)/intramammary | thymol | milk, plasma, liver, kidney, fat | [65] |
| dairy cattle | Phyto-Mast (essential oil of Thymus vulgaris and oregano)/intramammary | thymol | milk, plasma, liver, kidney, fat | [66] |
| horse | Bronchipret (equivalent to 2–4 g thyme extract)/orally | thymol | plasma | [69] |
3. Bioavailability of Thymol Generally and in Rabbits as Model Animal
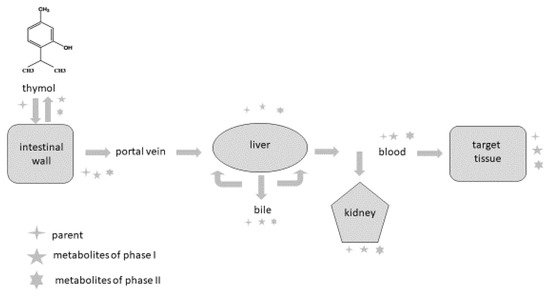
Phytogenic compounds and other foreign substances after oral administration are absorbed from the intestine, metabolised and eliminated from the organism. Once they reach the intestine or liver, they are converted during biotransformation processes (phase I and phase II) to more hydrophilic forms, and their pharmacological properties usually differ compared to the parental compound. It is also important to emphasise that metabolites can probably be deconjugated to the parental compound and express their pharmacological activity in this way. The major reactions occurring during phase I are oxidation, reduction and hydrolysis; phase II reactions are also called conjugation reactions and include glucuronidation, sulphation, acetylation, methylation, conjugation with glutathione and conjugation with amino acids [77,78,79,80,81,82].
The intestine plays an important role as a site for the absorption as well as biotransformation of thymol [63,76]. Thymol or its metabolites, after biotransformation processes in the intestinal wall, can be transported back into the intestinal lumen or are converted back to the parental compounds and redistributed within the organism through the systemic circulation [78]. Some part of the compounds are transported by the mesenteric vein into the liver, where they are metabolised, excreted into the duodenum in bile and again reabsorbed [57,78]. Bacova et al. [58] found 15% of thymol in the liver compared with the intestinal wall, which demonstrates the intensive absorption of thymol from the intestinal wall through the vena portae to the liver.
There are some barriers that the compounds must pass through during their metabolic path in the organism, and they limit their absorption [63, 79]. The first-pass metabolism in the intestine decreases the number of molecules reaching the blood circulation, and then the first-pass metabolism in the liver represents another barrier for the distribution of compounds in the organism. In addition to this, many efflux transporters are bound mainly with lipophilic molecules, which are rapidly excreted from the organism, greatly limiting their bioavailability [58,78,80,81,82]. These biotransformation processes are probably the reason why only a trace amount of thymol is found in the muscle and fat tissues of broilers [70,77] and rabbits [58].
The rabbit’s digestive processes represent a complex system of the separation of digestible and indigestible parts of ingested food in the proximal colon [83]. The most important mechanism by which nutrients are released from ingested food is microbial fermentation in the caecum. The products of fermentation are either absorbed directly through the caecal wall or are re-ingested as caecotrophs [84]. The caecum and colon are the most important parts of a rabbit’s digestive system in connection with the original feature of digestion, caecotrophy.
4. Conclusions
The presented data represent the available scientific information regarding the urgent need for more studies to precisely understand the metabolic processes and biological activity of thymol and its metabolites within organisms. This information will be useful for researchers, drug and pharmaceutical industries and the medical and veterinary sectors.
This entry is adapted from the peer-reviewed paper 10.3390/ani12091131
