1. Lysosomal Target of Nanoparticles (NPs) and Modulation of NPs for Lysosomal Function
1.1. pH Alteration
The primary function of the lysosome is the degradation of proteins and lipids [
1,
2]. The regulation of lysosomal pH has been linked to various cellular functions including the degradation of intracellular compartments. For its cellular functions, lysosomal lumen has to be maintained at an acidic pH [
3]. Degradation of proteins, which is a crucial function of the lysosome, is carried out by more than 60 kinds of lysosomal hydrolases [
4], and these hydrolases are optimized for the highly acidic environment of lysosomes (between pH 4.5 and 5.0) [
4,
5]. The lysosome as a cellular digestive system eliminates the garbage materials from autophagy and phagocytosis [
6,
7,
8]. Thus, destabilization of lysosomal pH thorough alkalization leads to cellular toxicity and even causes lysosomal storage disease (LSD) [
9,
10,
11]. The application of NPs can mediate various cellular functions by modulating lysosomal pH. Gold NPs (AuNPs) are known to reduce lysosomal activity by alkalization of the lysosomal lumen [
11]. This reaction triggers oxidative stress, mitochondrial damage, and decreases cell migration/invasion [
11]. In particular, 50-nm sized AuNPs induce autophagosomal accumulation of LC3 and block p62 degradation [
12]. Silver NPs (AgNPs) also suppress autophagic responses by decreasing transcription factor EB (TFEB) protein expression, which is followed by lysosomal alkalization [
13]. In addition, rare earth oxide NPs (REONPs)-mediated alkalization induces the activation of interleukin-1β IL-1β by an inflammasome [
14].
1.2. Cell Viability
The lysosome consists of a typical single phospholipid bilayer to control important cellular functions [
15,
16]. The lysosomal membrane acts as the connector to contact other compartments such as autophagosome [
17,
18], mitochondria [
19], and endoplasmic reticulum (ER) [
20]. On the lysosomal membrane, numerous proteins play important roles such as the mammalian target of rapamycin complex 1 (mTORC1) (nutrient sensing) [
21], V-ATPase (Vacuolar type of H
+-ATPase) (pH homeostasis) [
22], and ion channels/transporters [
23]. In addition, deficiency of several lysosomal membrane proteins trigger various diseases such as the Danon disease (lysosome associated membrane proteins, LAMP-2) [
24], malignant infantile osteopetrosis (the chloride channel 7, CLC-7) [
25], and actin myoclonus-renal failure syndrome (lysosomal integral membrane protein-2) [
26]. Damaged lysosome mediates lysosomal membrane permeabilization (LMP), which contributes to cell death [
27,
28] and induces several diseases such as LSD and other neurodegenerative disease [
29,
30,
31].
1.3. Protein Activity and Expression
Various lysosomal functions are mediated by more than 200 integral lysosomal membrane proteins [
4], including (1) the mechanistic target of mTORC1, which is activated by nutrient starvation [
28,
38], and acts as a negative regulator of autophagy [
28,
39], and (2) LAMPs, which protect the lysosomal membrane against lysosomal hydrolases not to degrade [
40]. NPs induce an inhibitory effect on the mTORC1 pathway to activate autophagy: AgNPs (decreases lysosomal protease activities) [
41], Zinc oxide (ZnO) NPs (induces macrophage cell death) [
42], and REONPs (induces lysosomal imbalance by TFEB nucleus translocation) [
43]. ZnO NPs induce an aberrant expression pattern and de-glycosylation of LAMP-2 by ZnO-induced reactive oxygen species (ROS), which trigger cell death in lung epithelial cells [
44]. Additionally, NPs modulate lysosomal motility [
45]. Lysosome movement reveals two directions: toward the peripheral cytoplasm (anterograde) [
46,
47] and juxtanuclear region (retrograde) [
48]. To carry out autophagic flux, lysosomes have to move to the juxtanuclear region [
22,
38], and the dynein complex is the motor protein for retrograde transport [
49]. Treatment with carbon nanotubes decreases the expression of synaptosomal-associated protein (SNAP), which is a regulating factor of dynein [
50] that blocks retrograde transport and, thus, the autophagic pathway [
45]. Taken together, the lysosomal pathways of NPs and occupied proteins may mediate numerous functions. Thus, careful and more extensive consideration of lysosomal-associated NPs needs to be done.
1.4. Accumulation of NPs
Toxic cellular components, such as cytoplasmic macromolecules, damaged or misfolded proteins, and other worn-out organelles, are removed by lysosomes to maintain metabolic homeostasis [
3]. Thus, the degradation role of lysosomes is essential for carrying out cellular homeostasis [
51] including lipid catabolism [
52], cell growth [
53], and neurotransmission [
54]. However, several NPs interrupt lysosomal degradation and deposit the lysosomal compartment in the cytoplasm. Exposure to AgNPs and copper oxide (CuO) NPs can induce agglomeration of lysosomes and subsequent cellular damage, which leads to cell death in human lung alveolar epithelial cells [
55] and human umbilical vein endothelial cells [
56]. In addition, NPs can accumulate in lysosomes. SiO
2NPs and PNPs impair cell viability and induce lysosomal swelling, which is followed by their accumulation in lysosomes and triggers lysosomal dysfunction and apoptosis [
57,
58].
2. Regulation of Lysosomal pH and Its Physiological Function
The lysosomal pH gradient is generated and maintained by movement of hydrogen ions (H
+) into the lysosomes through the action of vacuolar-type ATPases (V-ATPases) [
59], which is supplemented further by movement of other ions [
5]. Thus, for effective and continuous movement of H
+ into the lysosome, an accompanying counter-ion movement is necessary [
5].
The lysosomal V-ATPases consists of two domains: V
1 domain, which hydrolyses ATP, and the V
0 domain, which translocates H
+ ions across the lysosomal membrane [
60]. The catalytic domain V
1, drives a rotary H
+ transport motor by hydrolyzing ATP with translocation of H
+ [
61,
62]. In this case, the V-ATPase rotor is operated in only one direction with an irreversible ATP hydrolysis due to the movement of H
+ from cytosol to the lysosomal lumen [
5]. The continuous V-ATPase-mediated H
+ pumping generates a positive charge in the lysosomal lumen, which inhibits any further movement of H
+ [
63]. To dissipate this membrane potential, other ions have to be transferred in the opposite direction, and this process is referred to as the counterion flux [
5,
63]. Counter ion movement is suggested as both entering anions and exiting cations through the lysosomal lumen [
5]. One important counter ionic candidate is chloride, transferred by CLC-7, as attenuation of CLC-7 leads to lysosomal dysfunction such as LSD and osteopetrosis [
25,
64]. Another candidate counter ion is K
+, transferred by TMEM175. Its mutation induces neuronal degeneration and LSD [
65]. The R740S mutant osteoclasts, mutated in the V-ATPase α3 subunit, possess a higher lysosomal pH, and shows altered mTORC expression (increase in basal protein level and decrease of gene expression) and activity, which, in turn, plays a key role in cell proliferation [
57,
66]. Additionally, acidification of lysosomes can induce macrophages to secrete
N-acetyl-
β-D-glucosaminidase through lysosomal exocytosis [
67,
68], which includes absorption of cytochrome c in rat kidney during renal metabolism [
69], and transport of cystine, the product of protein degradation by cathepsin, from lysosomes to cytosol [
70]. Thus, alteration of lysosomal pH can be like a commander’s order to modulate the cellular life cycle.
3. Lysosome-Associated Ion Channels for Lysosomal Function
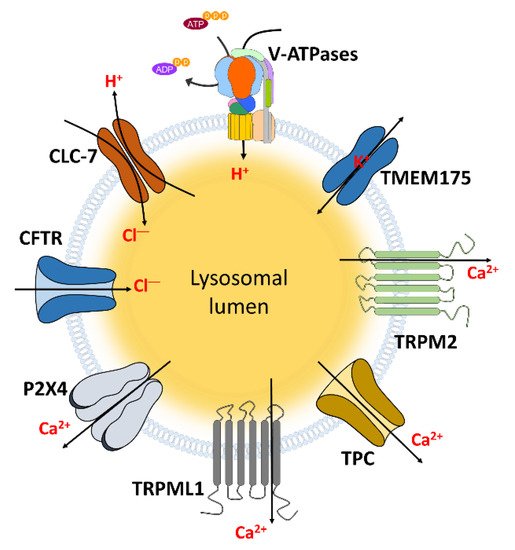
The lysosomal function is modulated by the ion movement and subsequent pH regulation. This movement is accomplished through various ion channels (Figure 1, Table 1).
Figure 1. The channels localized in lysosomal membrane to transport ions. These channels and transporters can regulate lysosomal and cellular functions through transporting and maintaining hydrogen, chloride, Ca2+, and potassium which indicated in Table 1.
Table 1. The relationship between lysosomal ion channels and cellular functions.
|
Channels
|
Mechanisms and Related Diseases
|
Ref.
|
|
CLC-3
|
Promotion of lysosomal acidification
|
[72,73]
|
|
CLC-6
|
LSD in CLC-6 mutated neuronal cells
|
[74]
|
|
CLC-7
|
Maintenance of acidic pH of lysosomes
|
[75,76]
|
|
Decrease of dentinogenesis and dental bone formation in CLC-7 deficient mice
|
[77,78]
|
|
Degradation of fAβ which drives AD
|
[79,80]
|
|
Osteopetrosis in CLC-7 mutation
|
[81,82,83,84]
|
|
LSD and neurodegeneration in CLC-7-deficient mice
|
[64,82]
|
|
CFTR
|
Support lysosomal acidification
|
[85]
|
|
Decrease of bacteria killing function and phago-lysosomal fusion in macrophage
|
[86]
|
|
TRPM2
|
Induce DC maturation and migration
|
[87]
|
|
Increase of actin remodeling
|
[88]
|
|
Increase of pancreatic β cell apoptosis
|
[89]
|
|
Increase LMP, NLRP3 inflammasome, and mitochondrial fission on the plasma membrane
|
[90,91]
|
|
TRPML1
|
Maintenance of acidic pH of lysosomes
|
[92]
|
|
Increase of large particle phagocytosis, bone remodeling, gastric acid secretion, and myocytes apoptosis
|
[93,94,95,96]
|
|
Stomach hypertrophy, hypergastrinemia, LSD, mucolipidosis, NPC, and AD in TRPML1 deficiency
|
[93,97,98,99,100,101,102,103]
|
|
TMEM175
|
Support lysosomal Ca2+ signaling and pH regulation
|
[104]
|
|
Related in LSD
|
[105]
|
|
TPC
|
Related in autophagy, cancer cell migration, and cellular pigmentation
|
[106,107,108]
|
|
Related in Parkinson’s disease
|
[109,110]
|
|
P2X4
|
Promotion of endo-lysosomal fusion
|
[111,112]
|
|
Related in liver fibrogenesis
|
[113]
|
Abbreviations: CLC: Chloride channel; CFTR: Cystic fibrosis transmembrane conductance regulator; TRPM2: Transient receptor potential melastatin 2; TRPML1: Transient receptor potential mucolipin 1; TMEM175: Transmembrane protein 175; TPC: Two pore channel; AD: Alzheimer’s disease; DC: dendritic cell; LMP: Lysosomal membrane permeabilization; NLRP3: NACHT, LRR and PYD domains-containing protein 3; NPC: Niemann-Pick disease type C.
4. NP-Induced Proton Sponge Effect through Ion Channels in the Tumor System
Swelling of lysosomes has the potential to increase cellular toxicity by releasing lysosomal compartments and nanoparticles [
187,
188]. The lysosomal ‘proton sponge effect’ is triggered by the influx of cationic nanoparticles with hydrogen and chloride ions to lysosomes [
188]. Accumulated ions in the lysosome may trigger water intake to equilibrate the physiological osmolarity and, subsequently, induce lysosomal rupture [
188]. It has been addressed that conceptual use of the lysosomal pH-dependent system and lysosomal rupture develops the self-assembled luminescent AuNPs by the swelling property [
189]. Lee et al. reported that encapsulated AuNR-DOX in lysosomes is dissociated with DOX by lysosomal hydrolases. A charged linker of AuNR is opened and then recruited negative charged ions such as chloride into the lysosome. The ionic accumulation is developed, and lysosomal rupture occurred. Released chloride from the lysosome through lysosomal rupture activates Ca
2+ influx channel TRPM2 in the plasma membrane and, lastly, overload of Ca
2+ triggers the enhanced apoptotic effect including the effect of DOX in cancer cells [
190]. The intracellular mechanism of nanomaterials and its related channels is now started. However, the effect of nanoparticles on lysosomal ion channels and transporters has still been poorly studied. To use nanomaterials for medicines, understanding the relationship between nanoparticles and lysosomal ion channels has to be expanded.
5. Clinical Application and Limitation of Nanomaterials
As mentioned earlier, NPs have a bio-toxic effect on lysosomes by triggering pH alteration, malfunctions of protein activity, accumulation in lysosomes, and subsequent cell death. The effect of NPs on cellular functions is summarized in Table 2. Accordingly, application of NPs has limitations for nanodrugs and nano-therapies. Thus, recent efforts have challenged to overcome these limitations by maximizing transport ability or reducing cytotoxicity.
Table 2. The effect of nanoparticles (NPs) on cellular functions.
|
Related Cellular Function
|
NPs
|
Details
|
Reference
|
|
pH alteration
(alkalization of lysosome)
|
AuNPs
|
Increase of oxidative stress, mitochondrial damage, and decrease cell migration/invasion
|
[11]
|
| |
Accumulation of LC3 and block p62 degradation
|
[12]
|
|
AgNPs
|
Decrease of TFEB protein expression
|
[13]
|
|
REONPs
|
Activation of IL-1β inflammasome
|
[14]
|
|
Cell viability
(cell death)
|
PNPs
|
Decrease of autophagic flux
|
[32]
|
| |
Decrease of cathepsin release
|
[34]
|
|
SiO2 NPs
|
Increase of membrane damage and NLRP inflammasome
|
[35,44]
|
|
TiO2 NPs
|
Increase of membrane damage
|
[36]
|
|
Gd2O3 NPs
|
Increase of membrane damage and necrosis
|
[37]
|
|
Protein activity and expression
|
AgNPs
|
Decrease of lysosomal protease activities
|
[41]
|
|
REONPs
|
Induce lysosomal imbalance by inhibiting mTORC1 pathway
|
[43]
|
|
ZnO NPs
|
Increase of macrophage cell death by inhibiting mTORC1 pathway
|
[42]
|
| |
Deglycosylation of LAMP-2
|
[44]
|
|
Carbon nanotube
|
Decrease of SNAP
|
[50]
|
|
Accumulation of NPs
|
CuO NPs
|
Subsequent cellular damage leading to cell death by agglomeration of lysosomes
|
[55,56]
|
|
SiO2 NPs, PNPs
|
Induce lysosomal swelling leading to apoptosis
|
[57,58]
|
Abbreviations: AuNP: Gold nanoparticle; AgNP: Silver nanoparticle; REONP: rare earth oxide nanoparticle; PNP: polystyrene nanoparticle; ZnO: Zinc oxide; CuO: Copper oxide; TFEB: Transcription factor EB; IL-1β: interleukin-1β; NLRP: NACHT, LRR and PYD domains-containing protein; mTORC1: rapamycin complex 1; SNAP: synaptosomal-associated protein.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics12030217