Eosinophils are innate immune granulocytes actively involved in defensive responses and in local and systemic inflammatory processes. Beyond these effector roles, eosinophils are fundamental to maintaining homeostasis in the tissues they reside. Gastrointestinal eosinophils modulate barrier function and mucosal immunity and promote tissue development through their direct communication with almost every cellular component. This is possible thanks to the variety of receptors they express and the bioactive molecules they store and release, including cytotoxic proteins, cytokines, growth factors, and neuropeptides and neurotrophines. A growing body of evidence points to the eosinophil as a key neuro-immune player in the regulation of gastrointestinal function, with potential implications in pathophysiological processes. Eosinophil–neuron interactions are facilitated by chemotaxis and adhesion molecules, and the mediators released may have excitatory or inhibitory effects on each cell type, with physiological consequences dependent on the type of innervation involved. Of special interest are the disorders of the brain–gut interaction (DBGIs), mainly functional dyspepsia (FD) and irritable bowel syndrome (IBS), in which mucosal eosinophilia and eosinophil activation have been identified.
- intestinal eosinophils
- neuro-immune interaction
- disorders of brain–gut interaction
1. Eosinophils Regulate the Intestinal Barrier Function
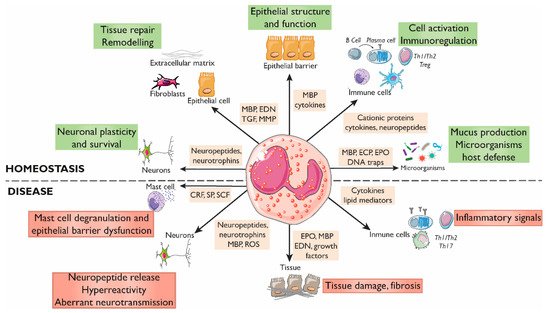
2. Eosinophils Modulate Intestinal Immune Responses
3. Eosinophil–Neuron Interactions
3.1. Innervation of the Gastrointestinal Tract
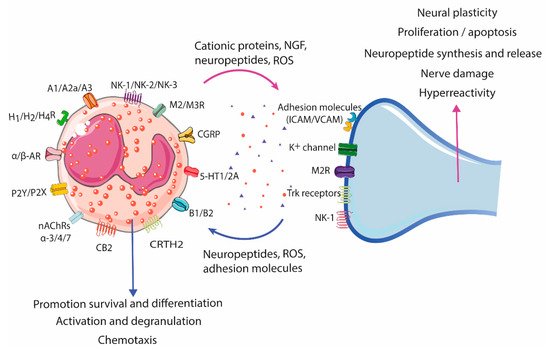
3.2. Neural-Induced Recruitment and Activation of Eosinophils by Extrinsic Nerves
3.3. Neural-Induced Recruitment and Activation of Eosinophils by the ENS
This entry is adapted from the peer-reviewed paper 10.3390/cells11101644
References
- Furuta, G.T.; Nieuwenhuis, E.E.S.; Karhausen, J.; Gleich, G.; Blumberg, R.S.; Lee, J.J.; Ackerman, S.J. Eosinophils Alter Colonic Epithelial Barrier Function: Role for Major Basic Protein. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 289, G890–G897.
- Jacoby, D.B.; Ueki, I.F.; Widdicombe, J.H.; Loegering, D.A.; Gleich, G.J.; Nadel, J.A. Effect of Human Eosinophil Major Basic Protein on Ion Transport in Dog Tracheal Epithelium. Am. Rev. Respir. Dis. 1988, 137, 13–16.
- White, S.R.; Sigrist, K.S.; Spaethe, S.M. Prostaglandin Secretion by Guinea Pig Tracheal Epithelial Cells Caused by Eosinophil Major Basic Protein. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1993, 265, L234–L242.
- Johnson, A.M.F.; Costanzo, A.; Gareau, M.G.; Armando, A.M.; Quehenberger, O.; Jameson, J.M.; Olefsky, J.M. High Fat Diet Causes Depletion of Intestinal Eosinophils Associated with Intestinal Permeability. PLoS ONE 2015, 10, e0122195.
- Furuta, G.T.; Katzka, D.A. Eosinophilic Esophagitis. N. Engl. J. Med. 2015, 373, 1640–1648.
- Mishra, A.; Hogan, S.P.; Lee, J.J.; Foster, P.S.; Rothenberg, M.E. Fundamental Signals That Regulate Eosinophil Homing to the Gastrointestinal Tract. J. Clin. Investig. 1999, 103, 1719–1727.
- Carlens, J.; Wahl, B.; Ballmaier, M.; Bulfone-Paus, S.; Förster, R.; Pabst, O. Common γ-Chain-Dependent Signals Confer Selective Survival of Eosinophils in the Murine Small Intestine. J. Immunol. 2009, 183, 5600–5607.
- Honda, K.; Chihara, J. Eosinophil Activation by Eotaxin--Eotaxin Primes the Production of Reactive Oxygen Species from Eosinophils. Allergy 1999, 54, 1262–1269.
- Choe, M.M.; Sporn, P.H.S.; Swartz, M.A. An in vitro Airway Wall Model of Remodeling. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2003, 285, L427–L433.
- Sherrill, J.D.; Kc, K.; Wu, D.; Djukic, Z.; Caldwell, J.M.; Stucke, E.M.; Kemme, K.A.; Costello, M.S.; Mingler, M.K.; Blanchard, C.; et al. Desmoglein-1 Regulates Esophageal Epithelial Barrier Function and Immune Responses in Eosinophilic Esophagitis. Mucosal Immunol. 2014, 7, 718–729.
- Racca, F.; Pellegatta, G.; Cataldo, G.; Vespa, E.; Carlani, E.; Pelaia, C.; Paoletti, G.; Messina, M.R.; Nappi, E.; Canonica, G.W.; et al. Type 2 Inflammation in Eosinophilic Esophagitis: From Pathophysiology to Therapeutic Targets. Front. Physiol. 2021, 12, 815842.
- Jung, Y.; Wen, T.; Mingler, M.K.; Caldwell, J.M.; Wang, Y.H.; Chaplin, D.D.; Lee, E.H.; Jang, M.H.; Woo, S.Y.; Seoh, J.Y.; et al. IL-1β in Eosinophil-Mediated Small Intestinal Homeostasis and IgA Production. Mucosal Immunol. 2015, 8, 930–942.
- Cohn, L.; Homer, R.J.; MacLeod, H.; Mohrs, M.; Brombacher, F.; Bottomly, K. Th2-Induced Airway Mucus Production Is Dependent on IL-4Ralpha, but Not on Eosinophils. J. Immunol. 1999, 162, 6178–6183.
- Burgel, P.-R.; Lazarus, S.C.; Tam, D.C.-W.; Ueki, I.F.; Atabai, K.; Birch, M.; Nadel, J.A. Human Eosinophils Induce Mucin Production in Airway Epithelial Cells Via Epidermal Growth Factor Receptor Activation. J. Immunol. 2001, 167, 5948–5954.
- Shen, H.; Xu, F.; Zhang, G.; Wang, S.; Xu, W. CCR3 Monoclonal Antibody Inhibits Airway Eosinophilic Inflammation and Mucus Overproduction in a Mouse Model of Asthma. Acta Pharmacol. Sin. 2006, 27, 1594–1599.
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like Release of Mitochondrial DNA by Eosinophils Contributes to Antibacterial Defense. Nat. Med. 2008, 14, 949–953.
- Mukherjee, M.; Lacy, P.; Ueki, S. Eosinophil Extracellular Traps and Inflammatory Pathologies—Untangling the Web! Front. Immunol. 2018, 9, 2763.
- Puxeddu, I.; Ribatti, D.; Crivellato, E.; Levi-Schaffer, F. Mast Cells and Eosinophils: A Novel Link between Inflammation and Angiogenesis in Allergic Diseases. J. Allergy Clin. Immunol. 2005, 116, 531–536.
- Gomes, I.; Mathur, S.K.; Espenshade, B.M.; Mori, Y.; Varga, J.; Ackerman, S.J. Eosinophil-Fibroblast Interactions Induce Fibroblast IL-6 Secretion and Extracellular Matrix Gene Expression: Implications in Fibrogenesis. J. Allergy Clin. Immunol. 2005, 116, 796–804.
- Temkin, V.; Aingorn, H.; Puxeddu, I.; Goldshmidt, O.; Zcharia, E.; Gleich, G.J.; Vlodavsky, I.; Levi-Schaffer, F. Eosinophil Major Basic Protein: First Identified Natural Heparanase-Inhibiting Protein. J. Allergy Clin. Immunol. 2004, 113, 703–709.
- Lee, J.J.; Jacobsen, E.A.; McGarry, M.P.; Schleimer, R.P.; Lee, N.A. Eosinophils in Health and Disease: The LIAR Hypothesis: Eosinophils and the LIAR Hypothesis. Clin. Exp. Allergy 2010, 40, 563–575.
- Shinkai, K.; Mohrs, M.; Locksley, R.M. Helper T Cells Regulate Type-2 Innate Immunity in vivo. Nature 2002, 420, 825–829.
- Wang, Y.-H.; Angkasekwinai, P.; Lu, N.; Voo, K.S.; Arima, K.; Hanabuchi, S.; Hippe, A.; Corrigan, C.J.; Dong, C.; Homey, B.; et al. IL-25 Augments Type 2 Immune Responses by Enhancing the Expansion and Functions of TSLP-DC–Activated Th2 Memory Cells. J. Exp. Med. 2007, 204, 1837–1847.
- Spencer, L.A.; Szela, C.T.; Perez, S.A.C.; Kirchhoffer, C.L.; Neves, J.S.; Radke, A.L.; Weller, P.F. Human Eosinophils Constitutively Express Multiple Th1, Th2, and Immunoregulatory Cytokines That Are Secreted Rapidly and Differentially. J. Leukoc. Biol. 2008, 85, 117–123.
- Akuthota, P.; Wang, H.B.; Spencer, L.A.; Weller, P.F. Immunoregulatory Roles of Eosinophils: A New Look at a Familiar Cell. Clin. Exp. Allergy 2008, 38, 1254–1263.
- Xenakis, J.J.; Howard, E.D.; Smith, K.M.; Olbrich, C.L.; Huang, Y.; Anketell, D.; Maldonado, S.; Cornwell, E.W.; Spencer, L.A. Resident Intestinal Eosinophils Constitutively Express Antigen Presentation Markers, and Include Two Phenotypically Distinct Subsets of Eosinophils. Immunology 2018, 154, 298–308.
- Farhan, R.K.; Vickers, M.A.; Ghaemmaghami, A.M.; Hall, A.M.; Barker, R.N.; Walsh, G.M. Effective Antigen Presentation to Helper T Cells by Human Eosinophils. Immunology 2016, 149, 413–422.
- Smith, K.M.; Rahman, R.S.; Spencer, L.A. Humoral Immunity Provides Resident Intestinal Eosinophils Access to Luminal Antigen via Eosinophil-Expressed Low-Affinity Fcγ Receptors. J. Immunol. 2016, 197, 3716–3724.
- Chu, D.K.; Jimenez-Saiz, R.; Verschoor, C.P.; Walker, T.D.; Goncharova, S.; Llop-Guevara, A.; Shen, P.; Gordon, M.E.; Barra, N.G.; Bassett, J.D.; et al. Indigenous Enteric Eosinophils Control DCs to Initiate a Primary Th2 Immune Response in vivo. J. Exp. Med. 2014, 211, 1657–1672.
- Wang, H.-B.; Weller, P.F. Pivotal Advance: Eosinophils Mediate Early Alum Adjuvant-Elicited B Cell Priming and IgM Production. J. Leukoc. Biol. 2008, 83, 817–821.
- Chu, V.T.; Beller, A.; Rausch, S.; Strandmark, J.; Zänker, M.; Arbach, O.; Kruglov, A.; Berek, C. Eosinophils Promote Generation and Maintenance of Immunoglobulin-A-Expressing Plasma Cells and Contribute to Gut Immune Homeostasis. Immunity 2014, 40, 582–593.
- Shakoory, B.; Fitzgerald, S.M.; Lee, S.A.; Chi, D.S.; Krishnaswamy, G. The Role of Human Mast Cell-Derived Cytokines in Eosinophil Biology. J. Interferon Cytokine Res. 2004, 24, 271–281.
- Galdiero, M.R.; Varricchi, G.; Seaf, M.; Marone, G.; Levi-Schaffer, F.; Marone, G. Bidirectional Mast Cell–Eosinophil Interactions in Inflammatory Disorders and Cancer. Front. Med. 2017, 4, 103.
- Niranjan, R.; Mavi, P.; Rayapudi, M.; Dynda, S.; Mishra, A. Pathogenic Role of Mast Cells in Experimental Eosinophilic Esophagitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G1087–G1094.
- Hirota, C.L.; McKay, D.M. Cholinergic Regulation of Epithelial Ion Transport in the Mammalian Intestine. Br. J. Pharmacol. 2006, 149, 463–479.
- Brierley, S.M.; Linden, D.R. Neuroplasticity and Dysfunction after Gastrointestinal Inflammation. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 611–627.
- Brinkman, D.J.; ten Hove, A.S.; Vervoordeldonk, M.J.; Luyer, M.D.; de Jonge, W.J. Neuroimmune Interactions in the Gut and Their Significance for Intestinal Immunity. Cells 2019, 8, 670.
- Veiga-Fernandes, H.; Mucida, D. Neuro-Immune Interactions at Barrier Surfaces. Cell 2016, 165, 801–811.
- Jacobson, A.; Yang, D.; Vella, M.; Chiu, I.M. The Intestinal Neuro-Immune Axis: Crosstalk between Neurons, Immune Cells, and Microbes. Mucosal Immunol. 2021, 14, 555–565.
- Chou, D.L.; Daugherty, B.L.; McKenna, E.K.; Hsu, W.M.; Tyler, N.K.; Plopper, C.G.; Hyde, D.M.; Schelegle, E.S.; Gershwin, L.J.; Miller, L.A. Chronic Aeroallergen during Infancy Enhances Eotaxin-3 Expression in Airway Epithelium and Nerves. Am. J. Respir. Cell Mol. Biol. 2005, 33, 1–8.
- Fryer, A.D. Neuronal Eotaxin and the Effects of Ccr3 Antagonist on Airway Hyperreactivity and M2 Receptor Dysfunction. J. Clin. Investig. 2005, 116, 228–236.
- Numao, T.; Agrawal, D.K. Neuropeptides Modulate Human Eosinophil Chemotaxis. J. Immunol. 1992, 149, 3309–3315.
- Jacoby, D.B.; Costello, R.M.; Fryer, A.D. Eosinophil Recruitment to the Airway Nerves. J. Allergy Clin. Immunol. 2001, 107, 211–218.
- Dunzendorfer, S.; Meierhofer, C.; Wiedermann, C.J. Signaling in Neuropeptide-Induced Migration of Human Eosinophils. J. Leukoc. Biol. 1998, 64, 828–834.
- Kingham, P.J.; McLean, W.G.; Walsh, M.-T.; Fryer, A.D.; Gleich, G.J.; Costello, R.W. Effects of Eosinophils on Nerve Cell Morphology and Development: The Role of Reactive Oxygen Species and P38 MAP Kinase. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2003, 285, L915–L924.
- Sawatzky, D.A.; Kingham, P.J.; Court, E.; Kumaravel, B.; Fryer, A.D.; Jacoby, D.B.; McLean, W.G.; Costello, R.W. Eosinophil Adhesion to Cholinergic Nerves via ICAM-1 and VCAM-1 and Associated Eosinophil Degranulation. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2002, 282, L1279–L1288.
- Nie, Z.; Nelson, C.S.; Jacoby, D.B.; Fryer, A.D. Expression and Regulation of Intercellular Adhesion Molecule-1 on Airway Parasympathetic Nerves. J. Allergy Clin. Immunol. 2007, 119, 1415–1422.
- Walsh, M.-T.; Curran, D.R.; Kingham, P.J.; Morgan, R.K.; Durcan, N.; Gleich, G.J.; McLean, W.G.; Costello, R.W. Effect of Eosinophil Adhesion on Intracellular Signaling in Cholinergic Nerve Cells. Am. J. Respir. Cell Mol. Biol. 2004, 30, 333–341.
- Kingham, P.J.; McLean, W.G.; Sawatzky, D.A.; Walsh, M.T.; Costello, R.W. Adhesion-Dependent Interactions between Eosinophils and Cholinergic Nerves. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2002, 282, L1229–L1238.
- Taylor-Clark, T.E.; Undem, B.J. Sensing Pulmonary Oxidative Stress by Lung Vagal Afferents. Respir. Physiol. Neurobiol. 2011, 178, 406–413.
- Evans, C.M.; Belmonte, K.E.; Costello, R.W.; Jacoby, D.B.; Gleich, G.J.; Fryer, A.D. Substance P-Induced Airway Hyperreactivity Is Mediated by Neuronal M 2 Receptor Dysfunction. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000, 279, L477–L486.
- Raap, M.; Rüdrich, U.; Ständer, S.; Gehring, M.; Kapp, A.; Raap, U. Substance P Activates Human Eosinophils. Exp. Dermatol. 2015, 24, 557–559.
- Smyth, C.M.; Akasheh, N.; Woods, S.; Kay, E.; Morgan, R.K.; Thornton, M.A.; O’Grady, A.; Cummins, R.; Sheils, O.; Smyth, P.; et al. Activated Eosinophils in Association with Enteric Nerves in Inflammatory Bowel Disease. PLoS ONE 2013, 8, e64216.
- O’Brien, L.M.; Fitzpatrick, E.; Baird, A.W.; Campion, D.P. Eosinophil–Nerve Interactions and Neuronal Plasticity in Rat Gut Associated Lymphoid Tissue (GALT) in Response to Enteric Parasitism. J. Neuroimmunol. 2008, 197, 1–9.
- Wallon, C.; Persborn, M.; Jönsson, M.; Wang, A.; Phan, V.; Lampinen, M.; Vicario, M.; Santos, J.; Sherman, P.M.; Carlson, M.; et al. Eosinophils Express Muscarinic Receptors and Corticotropin-Releasing Factor to Disrupt the Mucosal Barrier in Ulcerative Colitis. Gastroenterology 2011, 140, 1597–1607.
- Zheng, P.-Y.; Feng, B.-S.; Oluwole, C.; Struiksma, S.; Chen, X.; Li, P.; Tang, S.-G.; Yang, P.-C. Psychological Stress Induces Eosinophils to Produce Corticotrophin Releasing Hormone in the Intestine. Gut 2009, 58, 1473–1479.
- Kiank, C.; Taché, Y.; Larauche, M. Stress-Related Modulation of Inflammation in Experimental Models of Bowel Disease and Post-Infectious Irritable Bowel Syndrome: Role of Corticotropin-Releasing Factor Receptors. Brain Behav. Immun. 2010, 24, 41–48.
- Salvo-Romero, E.; Martínez, C.; Lobo, B.; Rodiño-Janeiro, B.K.; Pigrau, M.; Sánchez-Chardi, A.D.; González-Castro, A.M.; Fortea, M.; Pardo-Camacho, C.; Nieto, A.; et al. Overexpression of Corticotropin-Releasing Factor in Intestinal Mucosal Eosinophils Is Associated with Clinical Severity in Diarrhea-Predominant Irritable Bowel Syndrome. Sci. Rep. 2020, 10, 20706.
- Neunlist, M.; Toumi, F.; Oreschkova, T.; Denis, M.; Leborgne, J.; Laboisse, C.L.; Galmiche, J.P.; Jarry, A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G1028–G1036.
- Verma, A.K.; Manohar, M.; Venkateshaiah, S.U.; Mishra, A. Neuroendocrine cells derived chemokine vasoactive intestinal polypeptide (VIP) in allergic diseases. Cytokine Growth Factor Rev. 2017, 38, 37–48.
- El-Shazly, A.E.; Begon, D.Y.; Kustermans, G.; Arafa, M.; Dortu, E.; Henket, M.; Lefebvre, P.P.; Louis, R.; Delvenne, P. Novel Association between Vasoactive Intestinal Peptide and CRTH2 Receptor in Recruiting Eosinophils. J. Biol. Chem. 2013, 288, 1374–1384.
- Metwali, A.; Blum, A.M.; Ferraris, L.; Klein, J.S.; Fiocchi, C.; Weinstock, J.V. Eosinophils within the healthy or inflamed human intestine produce substance P and vasoactive intestinal peptide. J. Neuroimmunol. 1994, 52, 69–78.
