When malabsorbed carbohydrates reach the colon, they are fermented by colonic bacteria, with the production of short-chain fatty acids and gas lowering colonic pH. The appearance of diarrhoea or symptoms of flatulence depends in part on the balance between the production and elimination of these fermentation products. Different studies have shown that there are no differences in the frequency of sugar malabsorption between patients with irritable bowel disease (IBS) and healthy controls; however, the severity of symptoms after a sugar challenge is higher in patients than in controls.
1. Introduction
While, under normal conditions, most of an ingested carbohydrate load is completely absorbed before reaching the colon, several conditions can result in the impairment of absorption in the small intestine. Carbohydrate malabsorption can provoke an osmotically driven influx of fluid into the small bowel, leading to intestinal distension and rapid propulsion into the colon
[1]. In addition, unabsorbed carbohydrates are rapidly fermented by colonic microbiota, generating gas, lactate, and short-chain fatty acids that have effects on gastrointestinal function (
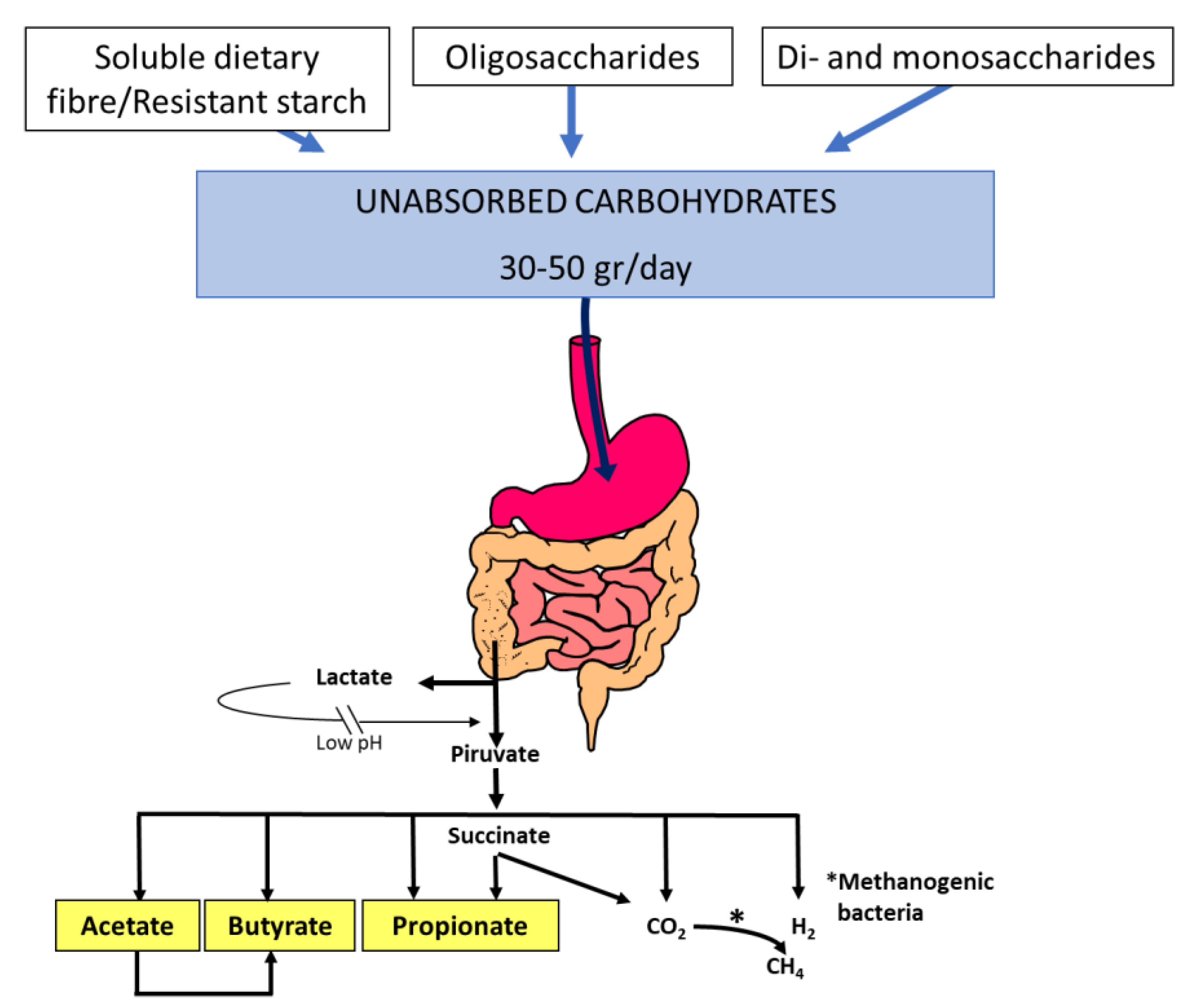
Figure 1)
[2]. All of these factors can produce diarrhoea, gas, bloating, flatulence, and abdominal pain, which are symptoms that patients with carbohydrate intolerance usually report. Other factors involving the development of symptoms are: 1. the quantity and quality of the ingested carbohydrate load; 2. the rate of gastric emptying; 3. the response of the small intestine to an osmotic load; 4. gastrointestinal motility; 5. the metabolic capacity of the colonic microbiota; and 6. the compensatory capacity of the colon to reabsorb water and short-chain fatty acids
[3].
Figure 1. Short-chain fatty acid and gas (H2, CO2, CH4) production by the colonic microbiota fermentation of dietary unabsorbed carbohydrates. Thirty to fifty grams of soluble dietary fibre, resistant starch, oligosaccharides (fructo-oligosaccharides, galacto-oligosaccharides, raffinose, and stachyose), disaccharides (lactose, sucrose), and monosaccharides (fructose) enter the colon each day and become available for colonic fermentation by the microbiota. Acetate (C2), propionate (C3), and butyrate (C4) are the main short-chain fatty acids that play important roles in gastrointestinal function.
2. Lactose Malabsorption and Intolerance
LM is typically caused by lactase downregulation after infancy due to lactase non-persistence, which, in Caucasians, is mediated by the LCT−13910:C/C genotype [4]. LM refers to an inefficient digestion of lactose in the small intestine. There are primary and secondary causes (such as viral gastroenteritis, giardiasis, and celiac disease). Lactose intolerance is the occurrence of gastrointestinal symptoms such as bloating, borborygmi, flatulence, abdominal pain, and diarrhoea in patients with LM after ingestion of lactose. The difference between LM and lactose intolerance is important since a lactose-restricted diet is only indicated in patients with both malabsorption and intolerance [5].
Symptoms of lactose intolerance characteristically do not arise until there is less than 50% of lactase activity. However, since adults with lactase deficiency often maintain between 10% and 30% of intestinal lactase activity, symptoms only develop when they eat enough lactose to overcome the compensatory mechanisms of the colon. There appears to be a dose-dependent effect.
HBT is the test of choice to assess LM and symptoms of intolerance [6][7][8]. Lactose HBT measures hydrogen produced by intestinal bacteria in the end-expiratory air after an oral challenge with a standard dose of lactose. In clinical practice, an intermediate lactose dose of 20–25 g in a 10% water solution is recommended [6][7][8][9].
3. Sucrase-Isomaltase Deficiency
Sucrose, or saccharose, is commercially known as cane sugar or regular table sugar and consists of one glucose and one fructose molecule. The bond between these two molecules is broken by the membrane-bound enzyme sucrase-isomaltase. The same enzyme also hydrolyses the glucose molecules in the short oligosaccharides and starch
[10]. Congenital sucrase-isomaltase deficiency (CSID) is a rare autosomal recessive condition with mutations of the sucrase-isomaltase gene on chromosome 3q25-26
[11]. Sucrase activity in intestinal villi is practically non-existent. The prevalence of CSID varies but has been described as 5–10% in Greenland, 3–7% in Canada, and 3% in Alaska. The prevalence in North America and Europe varies between 1/500 and 1/2000
[11]. Acquired forms of sucrase-isomaltase deficiency may be secondary to other chronic gastrointestinal conditions associated with intestinal villous atrophy, such as enteric infection, celiac disease, Crohn’s disease, and other enteropathies affecting the small intestine.
The symptoms usually appear in childhood and do not manifest until sucrose is included in the diet, which usually results from the introduction of fruit in the diet. It can also manifest at birth if a child is fed with a milk formula containing sucrose. In some individuals, it appears in adulthood with symptoms suggestive of IBS. In these cases, a careful anamnesis of the patient and their parents usually reveals a lifelong history of intestinal symptoms. Symptoms in childhood and in adulthood are similar, but the consequences are more serious in children. Severe watery diarrhoea may occur after the ingestion of small amounts of sucrose, which can be accompanied by abdominal pain, bloating, growth retardation, and the rejection of sugary foods. Isomaltose as such is not consumed in the diet. However, this oligosaccharide is released in the hydrolysis of starch. Although some patients may have mild symptoms after the ingestion of starch, most tolerate it well, mainly due to the low osmotic power of the molecule of undigested isomaltose.
4. Fructose Malabsorption
Fructose is commonly obtained from sugar beets, sugar cane, and maize and is the sweetest of all natural sugars
[12]. Fructose can be present as a monosaccharide or as disaccharide sucrose in a one-to-one molecular ratio with glucose. In recent decades, the solubility and sweetness of fructose have been exploited by the food industry in the form of sweetener blends derived from the enzymatic conversion of starches, with the increasing popularity of the use of high-fructose corn syrup (HFCS), a mixture of glucose and fructose in monosaccharide form, which may be of concern if it contains more fructose than glucose. In addition, use of fructose enhances the flavour and physical appeal of many foods and beverages. Thus, fructose is used in place of sucrose and other carbohydrates to reduce the caloric content of dietetic products while conserving high-quality sweetening profiles
[13].
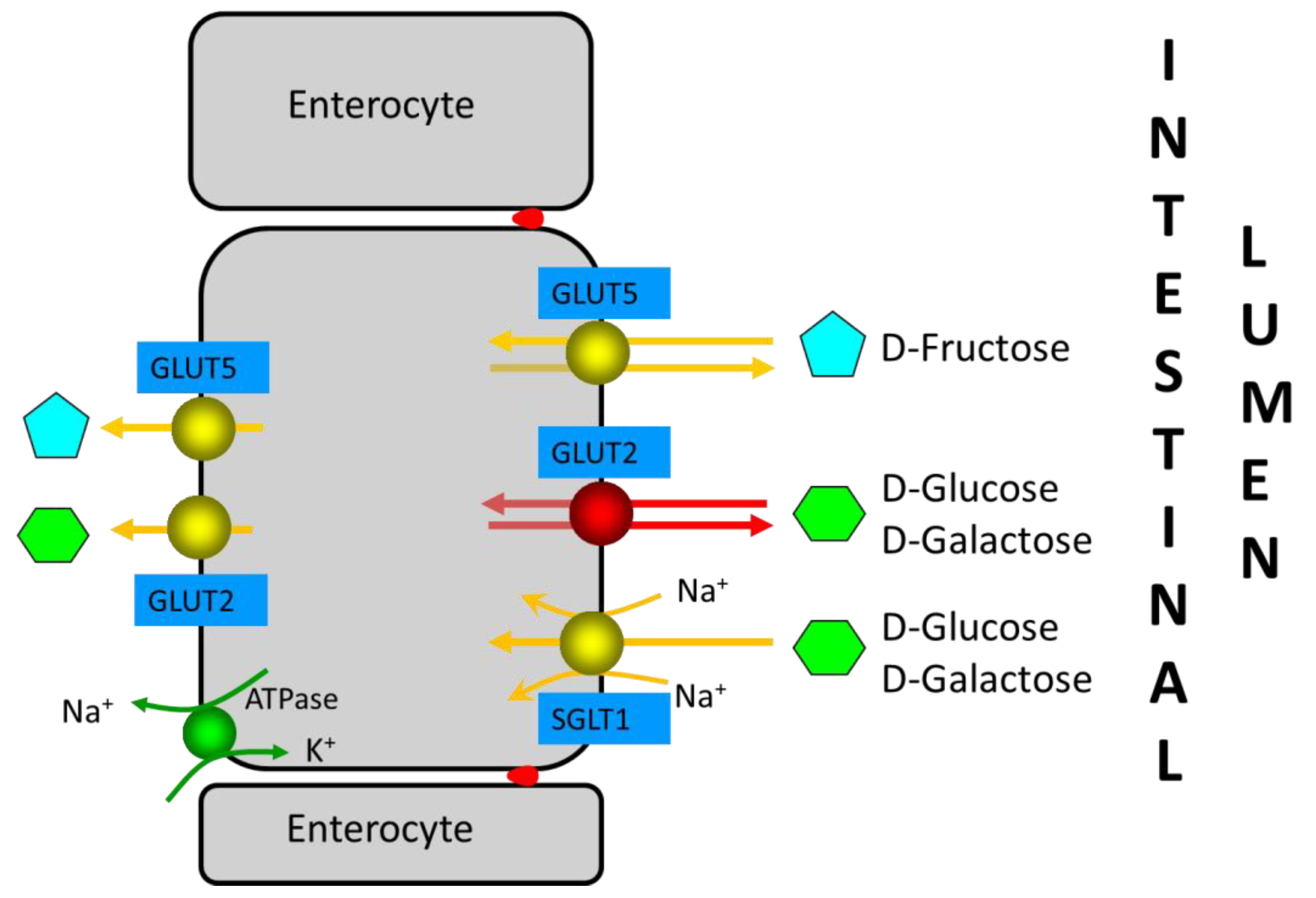
Fructose is mainly absorbed in the proximal small intestine. The absorption of monosaccharides is mainly mediated by the Na+-glucose cotransporter SGLT1 and the facilitative transporters GLUT2 and GLUT5
[14] (
Figure 2). In brief, SGLT1 and GLUT2 are important for the absorption of glucose and galactose, while the GLUT5 transporter is related to fructose absorption. SGLT1 and GLUT5 are continuously localized in the apical brush-border membrane of enterocytes, whereas GLUT2 is localized in the basolateral membrane at low luminal glucose concentrations or in both the apical brush-border membrane and the basolateral membrane at high luminal glucose concentrations. Fructose monosaccharide is transported across the apical intestinal brush-border membrane via a Na+-independent facilitated diffusion mechanism via the GLUT5 transporter. GLUT5 expression is induced by fructose, which exerts a fast and strong upregulation of GLUT5 mRNA expression, leading to an increase in GLUT5 protein and activity levels. Approximately 90% of the fructose entering the enterocyte is metabolized, increasing the intracellular pool of glucose. The remaining fructose subsequently exits the enterocytes to enter the blood via the GLUT2 and GLUT5 transporters present at the basolateral membrane. GLUT2 may be recruited transiently in the apical brush-border membrane in response to high luminal glucose concentrations to assist in the absorption of excess luminal fructose. GLUT2 is a high-capacity, low-affinity glucose/galactose transporter that can co-transport fructose in a one-to-one ratio. GLUT2 is unable to transport fructose without the presence of glucose, although the mechanism for this is currently unknown.
Figure 2. Enterocyte monosaccharide transporters involved in D-glucose, D-galactose, and D-fructose absorption in the small intestine. GLUT2, which is only observed in the apical brush-border membrane at high D-glucose concentrations in intestinal lumen, is indicated in red (see reference
[15]). Red dots between enterocytes represent the tight junctions.
Fructose malabsorption may be due to the insufficient uptake of fructose into enterocytes relative to the amount of luminal fructose. In addition, it may be caused by insufficient intracellular digestion of fructose, resulting in high intracellular fructose concentrations, which may contribute to decreased fructose uptake. In humans, fructose absorption capacity in the small intestine is much lower than glucose absorption capacity; it is very small after birth and increases later in response to dietary fructose. However, in combination with glucose, the capacity for fructose absorption increases due to the additional fructose uptake associated with Na+-glucose cotransport. Thus, fructose is well-absorbed in the presence of equimolar amounts of glucose in the proximal small intestine, while free fructose is absorbed slowly along the small intestine. Therefore, glucose co-ingestion significantly increases fructose absorption: glucose stimulates fructose absorption in a dose-dependent manner, and malabsorption occurs when fructose is present in excess of glucose. Unabsorbed fructose then passes into the colon and is fermented in the same manner as lactose in patients who have lactase non-persistence.
5. Sorbitol Intolerance
Sugar alcohols, a class of low-molecular weight polyols, can occur naturally or be obtained by the hydrogenation of sugars. The most common are sorbitol, mannitol, maltitol, isomalt, lactitol, and xylitol. Sorbitol (D-glucitol) is the most frequently consumed sugar alcohol. Small amounts of sorbitol are present in some fruits of the Rosaceae family (apples, pears, cherries, apricots, peaches, and prunes).
Sorbitol is poorly absorbed by the small intestine. It is well-known that, at high doses and concentrations, sorbitol is a laxative. Test solutions containing 10 g and 20 g resulted in 90% and 100%, respectively, of healthy volunteers showing malabsorption
[16]. After a 5 g dose administered at concentrations of 2%, 4%, 8%, and 16%, malabsorption was manifested in 10%, 12%, 22%, and 43% of healthy volunteers
[16]. Simultaneous ingestion of sorbitol and fructose seems to increase the malabsorption of the latter
[17][18]. Thus, polyol restriction has been incorporated as a part of a FODMAP (acronym for Fermentable, Oligo-Di- and Monosaccharides and Polyols) diet
[19].
6. Sugar Malabsorption and Functional Bowel Disease
Different studies have shown that there are no differences in the frequency of sugar malabsorption between patients with IBS and healthy controls, although the severity of symptoms after a sugar challenge is greater in patients than in controls
[9][20]. In a single-blind randomized controlled study in patients with diarrhoea-predominant IBS and healthy subjects
[20], sugar malabsorption was assessed by HBT after an oral load of various solutions containing lactose (50 g), fructose (25 g), sorbitol (5 g), fructose plus sorbitol (25 + 5 g), and sucrose (50 g). The frequency of sugar malabsorption was high in both patients and healthy controls, with malabsorption of at least one sugar solution in more than 90% of the subjects, but all subjects absorbed the sucrose solution. However, the symptoms score after both lactose and fructose plus sorbitol malabsorption was significantly higher in patients than in control subjects. In addition, more severe symptoms were observed in the IBS-D group after both lactose and fructose–sorbitol malabsorption than after the sucrose load administered as a control solution. Significantly more symptoms, although of mild intensity, were also observed after the sucrose load in patients with IBS than in healthy subjects. Finally, the administration of 10 g lactulose, a nonabsorbable carbohydrate, induced more symptoms and H
2 production in patients with IBS than in healthy controls. 40–50% of both patients with IBS and healthy controls malabsorbed the 25 g fructose load, and the severity of symptoms was no different between patients and controls.
7. Carbohydrate-Reduced Diets in Functional Bowel Disease
More recently, there has been renewed interest in evaluating the role of FODMAPs in patients with IBS and carbohydrate-related symptoms
[19]. FODMAPs are fermentable short-chain carbohydrates found in a variety of fruits, vegetables, pulses, dairy products, artificial sweeteners, and wheat. Evidence of a relationship between dietary FODMAPs and intestinal symptoms comes from a double-blind, cross-over study challenging patients with IBS with increasing doses of either glucose, fructose, fructans, or a mixture of the latter two for up to two weeks
[21]. Fructose or fructans were significantly more likely than glucose to induce symptom recurrence. A further cross-over study found that patients developed fewer symptoms after a low-FODMAP diet compared with a typical Australian diet
[22]. Afterwards, a randomized controlled trial found no differences between a low-FODMAP diet and an empiric IBS diet (NICE guidelines) based on healthy eating patterns, low fat content, and the avoidance of high-fibre food and resistant starch
[23]. Current British Society of Gastroenterology guidelines on the management of IBS recommend that a diet low in FODMAPs, which is considered to be a second-line dietary therapy, is an effective treatment for global symptoms and abdominal pain in IBS, although its implementation should be supervised by a trained dietitian, and fermentable oligosaccharides, disaccharides and monosaccharides, and polyols should be reintroduced according to tolerance (recommendation: weak, quality of evidence very low)
[24].
8. Mechanisms of Carbohydrate-Reduced Diet Improvement in Functional Bowel Disease
The mechanism by which dietary changes may affect symptoms in IBS patients was explored in a cross-over trial in which patients with IBS and bloating were recruited alongside a parallel cohort of healthy volunteers without functional gastrointestinal symptoms who followed the same trial regimen
[25]. Subjects were given 40 g of carbohydrate (glucose, fructose, and inulin in random order) in a 500-mL solution. Levels of breath hydrogen were measured, and intestinal content was assessed by MRI before and at various time points after the consumption of each drink. IBS patients and healthy subjects had similar physiological responses following fructose or inulin ingestion. These results indicate that colonic hypersensitivity to distension, rather than excessive gas production, produces the carbohydrate-related symptoms in IBS patients. Zhu et al.
[26] reported on lactose responsiveness in a Chinese population with a high prevalence of lactose maldigestion. They included IBS patients and healthy controls who underwent a 20 g lactose HBT, with assessments of hydrogen gas production and lactose intolerance symptoms. Lactose intolerance was more frequent in IBS than in healthy controls, especially bloating and borborygmus. Rectal hypersensitivity assessed by barostat was associated with a higher odds ratio of bloating than hydrogen production, suggesting that visceral hypersensitivity plays an important role in carbohydrate intolerance in IBS.
Therefore, osmotically active unabsorbed monosaccharides and disaccharides distend the small bowel with fluid and, subsequently, the colon, where they produce a gas increase and, in those subjects with visceral hypersensitivity, induce more severe gastrointestinal symptoms. Furthermore, the rapid colonic fermentation of unabsorbed carbohydrates generates gas and produces short-chain fatty acids, which lower colonic pH and trigger bowel symptoms
[27]. When delivered as liquid drinks, they speed gastric emptying, and the increase in small-bowel water content also accelerates intestinal transit, reducing small-bowel absorption, which may make symptoms more severe. Evidence that there are differences in visceral hypersensitivity in subsets of IBS patients suggests that the same magnitude of stimulus will produce different degrees of symptom response in patients depending on their sensory threshold
[24][28]. In the case of carbohydrate malabsorbers without IBS, symptom generation may be mainly triggered by rapid colonic fermentation.
This entry is adapted from the peer-reviewed paper 10.3390/nu14091923