The COVID-19 pandemic has affected many people in general and athletes in particular. This has led to a series of restrictions, which from a pathophysiological point of view, may affect the athlete’s performance in the short and long term. The restrictions basically affect training and eating habits, disturbing physical condition, as well as psychological behavior and general health status. Several aspects of systemic alterations caused by the SARS-CoV-2 virus and the resultant COVID-19 disease have been currently explored in the general population. Researchers believe that the most important element to take into account is the neuromuscular aspect, due to the implications that this system entails in motion execution and coordination. In this context, deficient neuromuscular control when performing dynamic actions can be an important risk factor for injury.
- athletes
- clinical consequences
- COVID-19
- physical activity
- return to sport
- SARS-CoV-2
1. Introduction
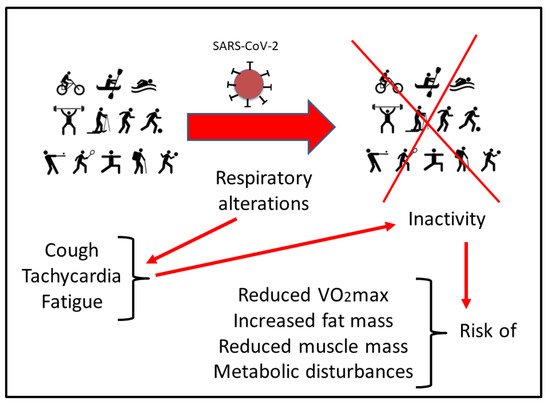
2. Respiratory Disturbances
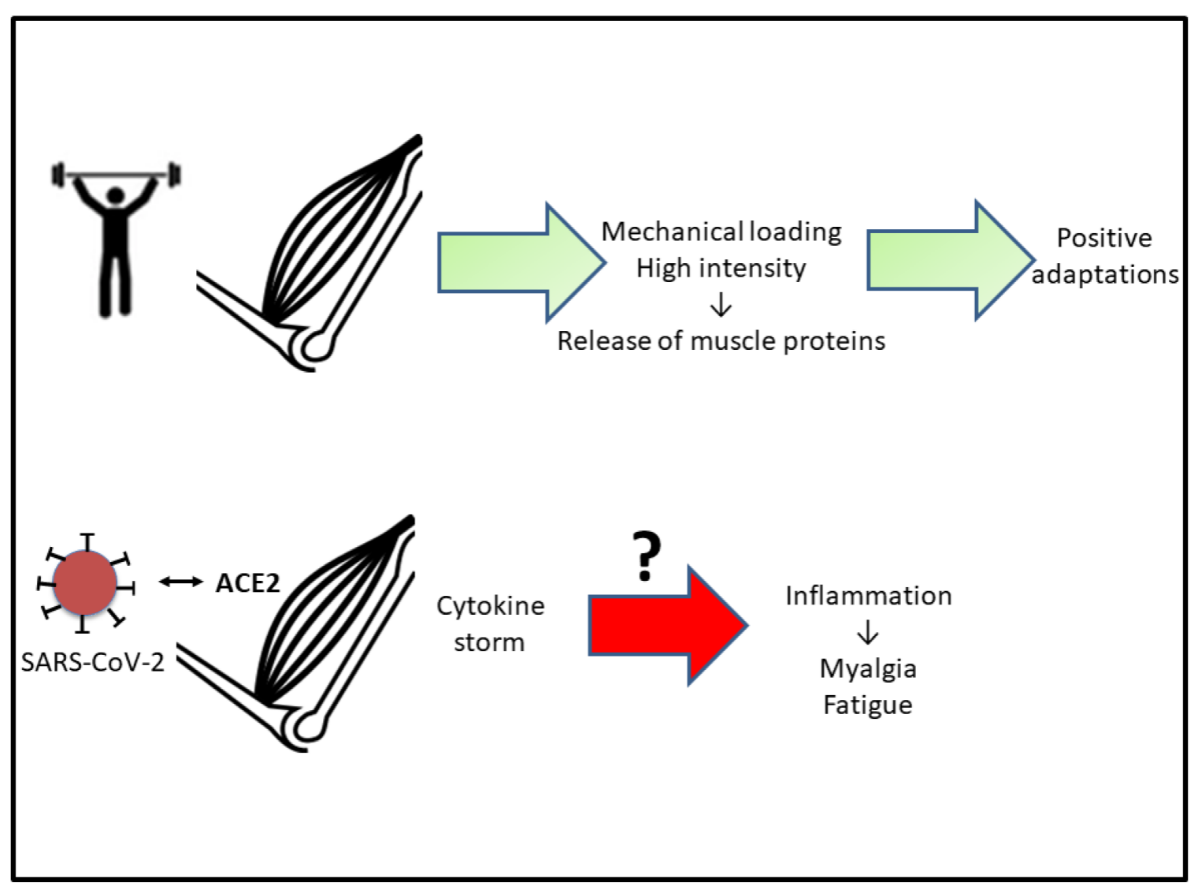
3. Muscular Repercussions
4. Cardiac Consequences
This entry is adapted from the peer-reviewed paper 10.3390/ijerph19095400
References
- Demarie, S.; Galvani, C.; Billat, V.L. Horse-Riding Competitions Pre and Post COVID-19: Effect of Anxiety, sRPE and HR on Performance in Eventing. Int. J. Environ. Res. Public Health 2020, 17, 8648.
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz Martínez, B.J.; Bernal-Morel, E.; Courel-Ibáñez, J. Post-COVID-19 syndrome and the potential benefits of exercise. Int. J. Environ. Res. Public Health 2021, 18, 5329.
- Dauty, M.; Menu, P.; Fouasson-Chailloux, A. Effects of the COVID-19 confinement period on physical conditions in young elite soccer players. J. Sports Med. Phys. Fit. 2021, 61, 1252–1257.
- Spyrou, K.; Alcaraz, P.E.; Marín-Cascales, E.; Herrero-Carrasco, R.; Cohen, D.D.; Calleja-Gonzalez, J.; Pereira, L.A.; Loturco, I.; Freitas, T.T. Effects of the COVID-19 Lockdown on Neuromuscular Performance and Body Composition in Elite Futsal Players. J. Strength Cond. Res. 2021, 35, 2309–2315.
- Font, R.; Irurtia, A.; Gutierrez, J.; Salas, S.; Vila, E.; Carmona, G. The effects of COVID-19 lockdown on jumping performance and aerobic capacity in elite handball players. Biol. Sport 2021, 38, 753–759.
- Corsini, A.; Bisciotti, G.N.; Eirale, C.; Volpi, P. Football cannot restart soon during the COVID-19 emergency! A critical perspective from the Italian experience and a call for action. Br. J. Sports Med. 2020, 54, 1186–1187.
- Brackbill, R.M.; Thorpe, L.E.; DiGrande, L.; Perrin, M.; Sapp, J.H.; Wu, D.; Campolucci, S.; Walker, D.J.; Cone, J.; Pulliam, P.; et al. Surveillance for World Trade Center Disaster Health Effects Among Survivors of Collapsed and Damaged Buildings. MMWR Surveill. Summ. 2006, 55, 1–18.
- Mills, M.A.; Edmondson, D.; Park, C.L. Trauma and Stress Response Among Hurricane Katrina Evacuees. Am. J. Public Health 2007, 97, S116–S123.
- Wang, C.; Pan, R.; Wan, X.; Tan, Y.; Xu, L.; Ho, C.S.; Ho, R.C. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int. J. Environ. Res. Public Health 2020, 17, 1729.
- Park, S.-C.; Park, Y.C. Mental Health Care Measures in Response to the 2019 Novel Coronavirus Outbreak in Korea. Psychiatry Investig. 2020, 17, 85–86.
- Martínez-Patiño, M.J.; Blas Lopez, F.J.; Dubois, M.; Vilain, E.; Fuentes-García, J.P. Effects of COVID-19 Home Confinement on Behavior, Perception of Threat, Stress and Training Patterns of Olympic and Paralympic Athletes. Int. J. Environ. Res. Public Health 2021, 18, 12780.
- Şenışık, S.; Denerel, N.; Köyağasıoğlu, O.; Tunç, S. The effect of isolation on athletes’ mental health during the COVID-19 pandemic. Phys. Sportsmed. 2021, 49, 187–193.
- Uroh, C.C.; Adewunmi, C.M. Psychological Impact of the COVID-19 Pandemic on Athletes. Front. Sports Act. Living 2021, 3, 603415.
- Melnyk, Y.B.; Stadnik, A.V.; Pypenko, I.S.; Kostina, V.V.; Yevtushenko, D.O. Impact of COVID-19 on the social and psychological state of athletes. J. Sports Med. Phys. Fit. 2022, 62, 297–299.
- Lima, Y.; Denerel, N.; Öz, N.D.; Senisik, S. The psychological impact of COVID-19 infection on athletes: Example of professional male football players. Sci. Med. Footb. 2021, 5, 53–61.
- Garfin, D.R.; Thompson, R.R.; Holman, E.A. Acute stress and subsequent health outcomes: A systematic review. J. Psychosom. Res. 2018, 112, 107–113.
- Kirwan, R.; McCullough, D.; Butler, T.; de Heredia, F.P.; Davies, I.G.; Stewart, C. Sarcopenia during COVID-19 lockdown restrictions: Long-term health effects of short-term muscle loss. Geroscience 2020, 42, 1547–1578.
- Palmer, K.; Monaco, A.; Kivipelto, M.; Onder, G.; Maggi, S.; Michel, J.-P.; Prieto, R.; Sykara, G.; Donde, S. The potential long-term impact of the COVID-19 outbreak on patients with non-communicable diseases in Europe: Consequences for healthy ageing. Aging Clin. Exp. Res. 2020, 32, 1189–1194.
- Magdy, D.M.; Metwally, A.; Tawab, D.A.; Hassan, S.A.; Makboul, M.; Farghaly, S. Long-term COVID-19 effects on pulmonary function, exercise capacity, and health status. Ann. Thorac. Med. 2022, 17, 28–36.
- Fabre, J.-B.; Grelot, L.; Vanbiervielt, W.; Mazerie, J.; Manca, R.; Martin, V. Managing the combined consequences of COVID-19 infection and lock-down policies on athletes: Narrative review and guidelines proposal for a safe return to sport. BMJ Open Sport Exerc. Med. 2020, 6, e000849.
- Córdova, A. Fisiología Deportiva; Síntesis: Madrid, Spain, 2013.
- Olsen, R.H.; Krogh-Madsen, R.; Thomsen, C.; Booth, F.W.; Pedersen, B.K. Metabolic Responses to Reduced Daily Steps in Healthy Nonexercising Men. JAMA 2008, 299, 1261–1263.
- Mikus, C.R.; Oberlin, D.J.; Libla, J.L.; Taylor, A.M.; Booth, F.W.; Thyfault, J.P. Lowering Physical Activity Impairs Glycemic Control in Healthy Volunteers. Med. Sci. Sports Exerc. 2012, 44, 225–231.
- Martinez-Ferran, M.; De La Guía-Galipienso, F.; Sanchis-Gomar, F.; Pareja-Galeano, H. Metabolic Impacts of Confinement during the COVID-19 Pandemic Due to Modified Diet and Physical Activity Habits. Nutrients 2020, 12, 1549.
- Wilson, M.G.; Hull, J.H.; Rogers, J.; Pollock, N.; Dodd, M.; Haines, J.; Harris, S.; Loosemore, M.; Malhotra, A.; Pieles, G.; et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: A practical guide for sport and exercise medicine physicians. Br. J. Sports Med. 2020, 54, 1157–1161.
- Astrand, P.O. Quantification of exercise capability and evaluation of physical capacity in man. Prog. Cardiovasc. Dis. 1976, 19, 51–67.
- Yu, C.; Li, A.M.; So, R.C.H.; McManus, A.; Ng, P.C.; Chu, W.; Chan, D.; Cheng, F.; Chiu, W.K.; Leung, C.W.; et al. Longer term follow up of aerobic capacity in children affected by severe acute respiratory syndrome (SARS). Thorax 2006, 61, 240–246.
- Narici, M.; De Vito, G.; Franchi, M.; Paoli, A.; Moro, T.; Marcolin, G.; Grassi, B.; Baldassarre, G.; Zuccarelli, L.; Biolo, G.; et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci. 2021, 21, 614–635.
- Gu, J.; Gong, E.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Zhan, J.; Wang, S.; Xie, Z.; et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424.
- Zhu, J.; Ji, P.; Pang, J.; Zhong, Z.; Li, H.; He, C.; Zhang, J.; Zhao, C. Clinical characteristics of 3062 COVID-19 patients: A meta analysis. J. Med. Virol. 2020, 92, 1902–1914.
- Cao, Y.; Liu, X.; Xiong, L.; Cai, K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J. Med. Virol. 2020, 92, 1449–1459.
- Kadoguchi, T.; Kinugawa, S.; Takada, S.; Fukushima, A.; Furihata, T.; Homma, T.; Masaki, Y.; Mizushima, W.; Nishikawa, M.; Takahashi, M.; et al. Angiotensin II can directly induce mitochondrial dysfunction, decrease oxidative fibre number and induce atrophy in mouse hindlimb skeletal muscle. Exp. Physiol. 2015, 100, 312–322.
- Kim, J.-H.; Thompson, L.V. Inactivity, age, and exercise: Single-muscle fiber power generation. J. Appl. Physiol. 2013, 114, 90–98.
- Jee, H.; Kim, J.-H. A mini-overview of single muscle fibre mechanics: The effects of age, inactivity and exercise in animals and humans. Swiss. Med. Wkly. 2017, 147, w14488.
- Rehman, T.; Josephson, G.; Sunbuli, M.; Chadaga, A.R. Spontaneous Pneumothorax in an Elderly Patient with Coronavirus Disease (COVID-19) Pneumonia. Ochsner J. 2020, 20, 343–345.
- Wang, J.-T.; Sheng, W.-H.; Fang, C.-T.; Chen, Y.-C.; Wang, J.-L.; Yu, C.-J.; Chang, S.-C.; Yang, P.-C. Clinical Manifestations, Laboratory Findings, and Treatment Outcomes of SARS Patients. Emerg. Infect. Dis. 2004, 10, 818–824.
- Chen, L.-L.; Hsu, C.-W.; Tian, Y.-C.; Fang, J.-T. Rhabdomyolysis associated with acute renal failure in patients with severe acute respiratory syndrome. Int. J. Clin. Pract. 2005, 59, 1162–1166.
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690.
- Kley, R.A.; Schmidt-Wilcke, T.; Vorgerd, M. Differential Diagnosis of HyperCKemia. Neurol. Int. Open 2018, 2, E72–E83.
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25.
- Córdova, A.; Martin, J.F.; Reyes, E.; Alvarez-Mon, M. Protection against muscle damage in competitive sports players: The effect of the immunomodulator AM3. J. Sports Sci. 2004, 22, 827–833.
- Cordova, A.; Monserrat, J.; Villa, G.; Reyes, E.; Soto, M.A.-M. Effects of AM3 (Inmunoferon) on increased serum concentrations of interleukin-6 and tumour necrosis factor receptors I and II in cyclists. J. Sports Sci. 2006, 24, 565–573.
- Córdova-Martínez, A.; Martorell-Pons, M.; Sureda-Gomila, A.; Tur-Marí, J.A.; Pons-Biescas, A. Changes in circulating cytokines and markers of muscle damage in elite cyclists during a multi-stage competition. Clin. Physiol. Funct. Imaging 2015, 35, 351–358.
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069.
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.C.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195.
- Cordova, A. Fisiología Dinámica; Ed Elsevier-Masson: Barcelona, Spain, 2003.
- Majano, P.; Roda-Navarro, P.; Alonso-Lebrero, J.L.; Brieva, A.; Casal, C.; Pivel, J.P.; López-Cabrera, M.; Moreno-Otero, R. AM3 inhibits HBV replication through activation of peripheral blood mononuclear cells. Int. Immunopharmacol. 2004, 4, 921–927.
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9.
- Draganidis, D.; Karagounis, L.G.; Athanailiidis, I.; Chatzinikolaou, A.; Jamurtas, A.Z.; Fatouros, I.G. Inflammaging and Skeletal Muscle: Can Protein Intake Make a Difference? J. Nutr. 2016, 146, 1940–1952.
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic markers in sports medicine. Adv. Clin. Chem. 2012, 56, 1–54.
- Linossier, M.-T.; Dormois, D.; Perier, C.; Frey, J.; Geyssant, A.; Denis, C. Enzyme adaptations of human skeletal muscle during bicycle short-sprint training and detraining. Acta Physiol. Scand. 1997, 161, 439–445.
- Dawson, B.; Fitzsimons, M.; Green, S.; Goodman, C.; Carey, M.; Cole, K. Changes in performance, muscle metabolites, enzymes and fibre types after short sprint training. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 78, 163–169.
- Izquierdo, M.; Ibañez, J.; Calbet, J.A.L.; González-Izal, M.; Navarro-Amézqueta, I.; Granados, C.; Malanda, A.; Idoate, F.; González-Badillo, J.J.; Häkkinen, K.; et al. Neuromuscular Fatigue after Resistance Training. Int. J. Sports Med. 2009, 30, 614–623.
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256.
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.R.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655.
- Paul, J.-F.; Charles, P.; Richaud, C.; Caussin, C.; Diakov, C. Myocarditis revealing COVID-19 infection in a young patient. Eur. Heart. J. Cardiovasc. Imaging 2020, 21, 776.
- Nieman, D.C. Is infection risk linked to exercise workload? Med. Sci. Sports Exerc. 2000, 32, S406–S411.
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22.
- Doyen, D.; Moceri, P.; Ducreux, D.; Dellamonica, J. Myocarditis in a patient with COVID-19: A cause of raised troponin and ECG changes. Lancet 2020, 395, 1516.
- Boraita-Pérez, A.; Serratosa-Fernández, L. Sudden death (IV). Sudden death in the athlete. The minimal requirements before performing a competitive sport. Rev. Esp. Cardiol. 1999, 52, 1139–1145.
- Emery, M.S.; Kovacs, R.J. Sudden Cardiac Death in Athletes. JACC Heart Fail. 2018, 6, 30–40.
- Corrado, D.; Zorzi, A. Sudden death in athletes. Int. J. Cardiol. 2017, 237, 67–70.
- Muñoz-Martínez, F.A.; Rubio-Arias, J.Á.; Ramos-Campo, D.J.; Alcaraz, P.E. Effectiveness of Resistance Circuit-Based Training for Maximum Oxygen Uptake and Upper-Body One-Repetition Maximum Improvements: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 2553–2568.
- Costill, D.L.; Fink, W.J.; Hargreaves, M.; King, D.S.; Thomas, R.; Fielding, R. Metabolic characteristics of skeletal muscle during detraining from competitive swimming. Med. Sci. Sports Exerc. 1985, 17, 339–343.
- Neufer, P.D.; Costill, D.L.; Fielding, R.A.; Flynn, M.G.; Kirwan, J.P. Effect of reduced training on muscular strength and endurance in competitive swimmers. Med. Sci. Sports Exerc. 1987, 19, 486–490.
- Demarie, S.; Chirico, E.; Galvani, C. Prediction and Analysis of Tokyo Olympic Games Swimming Results: Impact of the COVID-19 Pandemic on Swimmers’ Performance. Int. J. Environ. Res. Public Health 2022, 19, 2110.
- Rampinini, E.; Donghi, F.; Martin, M.; Bosio, A.; Riggio, M.; Maffiuletti, N.A. Impact of COVID-19 Lockdown on Serie a Soccer Players’ Physical Qualities. Int. J. Sports Med. 2021, 42, 917–923.
