The demand for mobile applications in agriculture is increasing as smartphones are continuously developed and used for many purposes; one of them is managing pests and diseases in crops. Using mobile applications, farmers can detect early infection and improve the specified treatment and precautions to prevent further infection from occurring. Furthermore, farmers can communicate with agricultural authorities to manage their farm from home, and efficiently obtain information such as the spectral signature of crops. Therefore, the spectral signature can be used as a reference to detect pests and diseases with a hyperspectral sensor more efficiently than the conventional method, which takes more time to monitor the entire crop field.
- crop
- disease
- mobile app
- pest
- spectral signature
1. Introduction
2. Mobile Application for Pest and Disease Management
2.1. Role of Mobile Applications in Monitoring Pest and Disease
| Name of Application | Function of Application | Country | Accuracy of Pest and/or Disease Identification | Reference |
|---|---|---|---|---|
| PlantifyAI | To diagnose 26 diseases across 14 crop species by offering treatment methods, common symptoms, and access to suggested cure treatments for each disease. | United States of America | Disease and crop classification: 95.7%. | Shrimali et al. [28] |
| Not mentioned | To identify and classify pests in images, extract characteristics of pests, and evaluate areas that prone to pests | Taiwan | Pest identification: 84%, and pest classification: 86% | Chen et al. [29] |
| Padi2U | To create a database of spectral signatures of weed species in rice fields | Malaysia | Weed separation species: 710 nm to 750 nm areas | Roslin et al. [30] |
| Mentha Mitra | To provide information about improved menthol mint types, nutrient requirements, diseases, and mechanisms for insect-pest control. | India | Not mentioned | Singh et al. [31] |
| Sistem Pakar Identifikasi Hama dan Penyakit Padi | To obtain a response from the user on the signs of pests and diseases that exist in rice | Indonesia | Not mentioned | Triono and Tristono [32] |
| e-RICE | To categorise the symptoms in order to make an accurate diagnosis of common rice diseases and problems. | Philippines | 4.29 rating by respondents agree that the app is functional in detecting disease | Morco et al. [33] |
| Dr Lada | To identify pests and diseases in peppers and propose appropriate techniques to solve the problem | Malaysia | Pest and disease diagnosis: 97% | Adama et al. [34] |
| PEST APP | To provide an early warning system on the infestation of the pest at early stages in paddy | Malaysia | Not mentioned | Nasir et al. [35] |
| Not mentioned | To identify the extend of cold-induced injuries in zucchini in real acquisition condition | Spain | Not mentioned | Novas et al. [36] |
| Leaf Analysis | To identify disease in different types of crop | Spain | Picon et al. [37] | |
| TobaccoApp | To detect any damage on tobacco leaf | Mexico | Damage caused by fungi: 97% | Valdez-Morones et al. [38] |
| Not mentioned | To control irrigation system and identify the images of plant leaf disease | India | Not mentioned | Ranjith et al. [39] |
| AuToDiDAC | To detect, separate, and assess the disease in cacao black pod rot | Philippines | Disease detection | Tan et al. [40] |
| cFertiGUAL | by calculating the amounts of fertiliser and monitoring irrigation systems, and select the best amongst the many crop growth systems and fertigation technologies | Spain | Disease detection: 97% | Pérez-Castro et al. [41] |
| FarmAR | To provide information about plants to farmers such as common name, scientific name of the plant, and plant diseases | Greece | Not mentioned | Katsaros and Keramopoulos [42] |
| Jaguza Livestock App | To improve the production and productivity of livestock by detecting livestock diseases and dealing with dangerous disease outbreaks. | Uganda | Not mentioned | Katamba and Mutebi [43] |
| BioLeaf | To quantify the foliar damage induced by insect herbivores on leaves | Brazil | Regular artificial damage: 25% and 50% of damaged area | Machado et al. [44] |
| Online at Sawah (OAS) | To detect diseases or pests that affect corn based on symptoms provided by users | Indonesia | Effectiveness: 82.5%, efficiency: 93.12%; learnability: 77.33%, and satisfaction: 73% | Simorangkir et al. [45] |
| Not mentioned | To identify the disease on wheat crop based on the detection of early symptom | Spain | Colour constancy algorithm of disease image: 0.81 | Johannes et al. [25] |
| Plant Disease | To diagnose plant disease with extensible set of diseases | Greece | Disease recognition: Between 80% and 98% | Petrellis [46] |
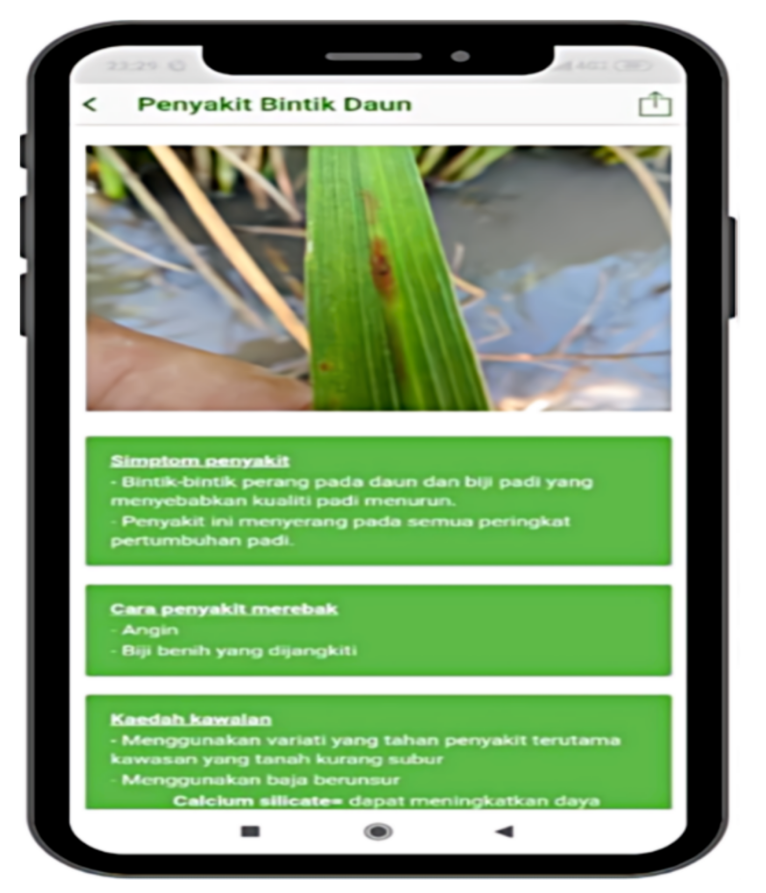
| Malay Language | English Language |
|---|---|
| Penyakit Bintik Daun | Lead spot disease |
| Simptom penyakit Bintik-bintik perang pada daun dan biji padi yang menyebabkan kualiti padi menurun. Penyakit ini menyerang pada semua peringkat pertumbuhan padi |
Symptoms of the disease Brown spots on the leaves and seeds of rice that cause the decline of rice quality. The disease attacks at all stages of rice growth. |
| Cara penyakit merebak Angin Biji benih yang dijangkiti |
Methods on the spread of disease Wind Infected seeds |
| Kaedah kawalan Menggunakan variati yang tahan penyakit terutama kawasan yang kurang subur. Menggunakan baja berunsur cancium sillicates |
Control methods Using disease-resistant varieties in less fertile areas Using calcium silicates fertilizers |
2.2. Image Processing for Pest and Disease Monitoring Using the Mobile Application
2.3. Systems for Extraction of Disease Using the Mobile Application
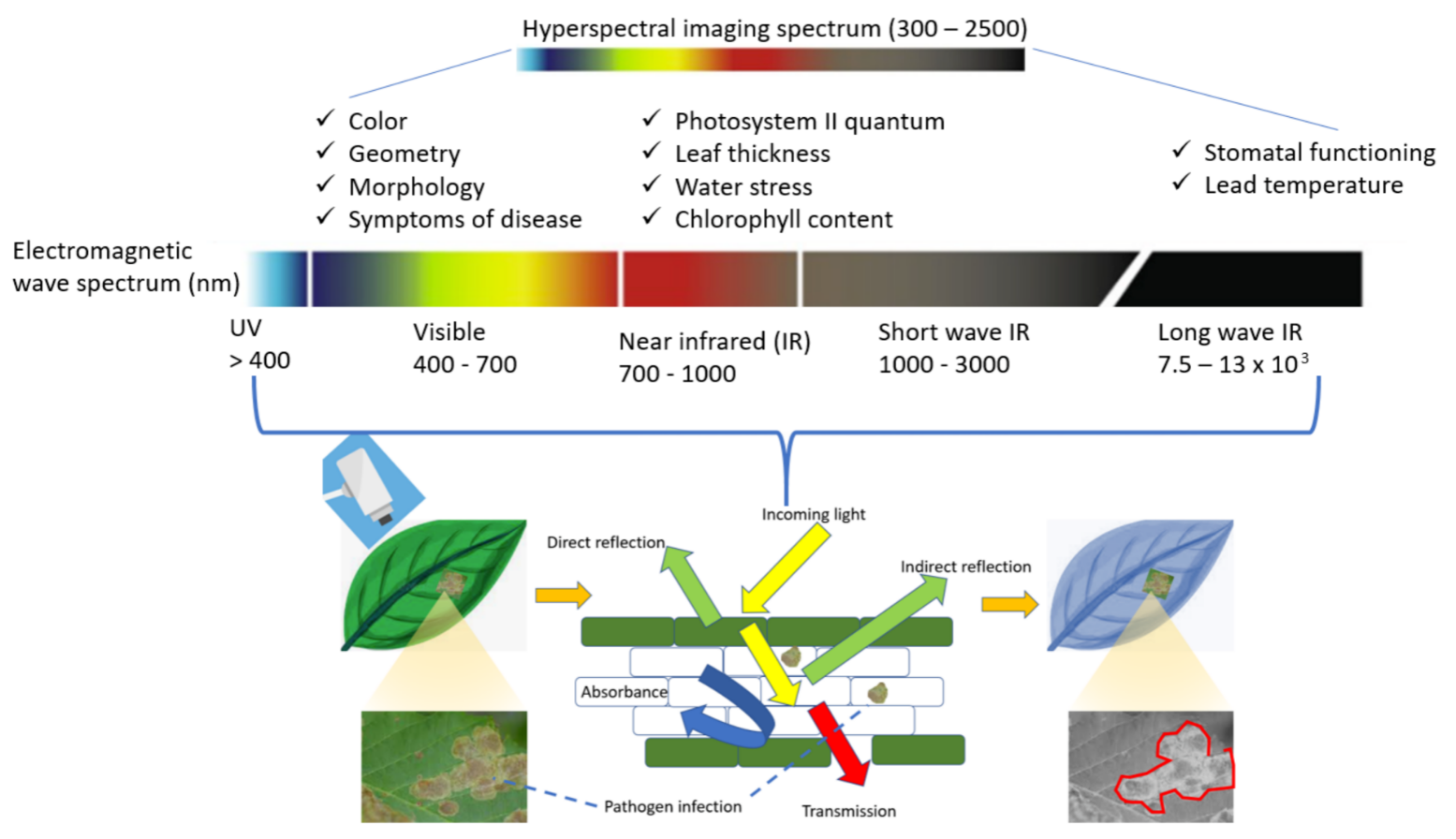
3. Spectral Signature Analysis for Pest and Disease Management
3.1. Spectral Reflectance in Monitoring Plant Health

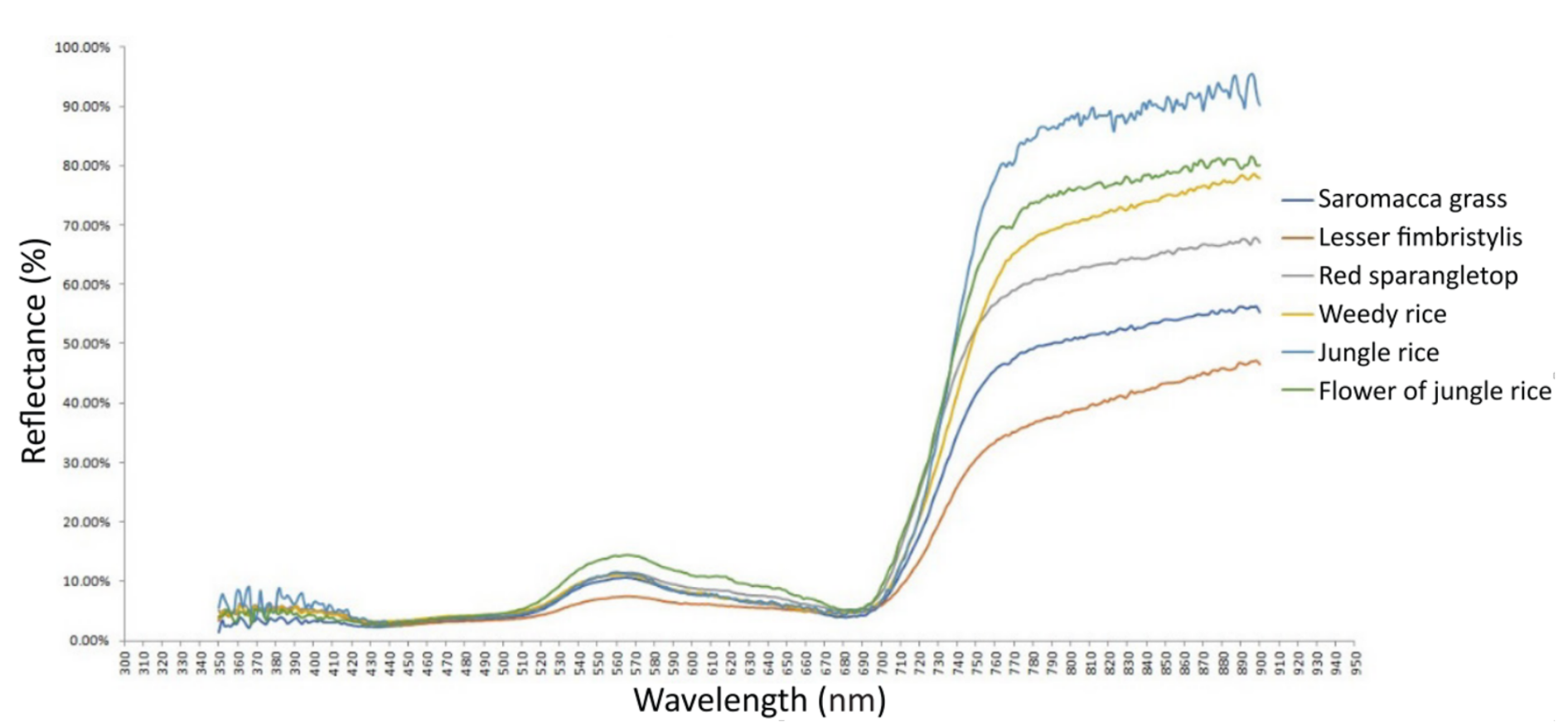
3.2. Spectral Signature of Pest and Diseases in the Crop Field
This entry is adapted from the peer-reviewed paper 10.3390/agronomy12040967
References
- Costopoulos, C.; Ntaliani, M.; Karetsos, S. Studying Mobile Apps for Agriculture. IOSR J. Mob. Comput. Appl. 2016, 3, 44–49.
- Bayrak, T. Identifying Technical Requirements for a Mobile Business Analytics Application. Int. J. Bus. Anal IJBAN 2021, 8, 91–103.
- Tudpor, K.; Wongkongdech, A.; Wongkongdech, R.; Chaiyakarm, T.; Jitsukka, W.; Sombateyotha, K.; Leethongdeesakul, S.; Kuboonya-Aragsa, N.; Chantarsombat, C.; Kimchai, K. Geographic Information System-Based Mobile Application Design for Health Care in Older Persons in Rural Community by Village Health Volunteers. Stud. Health Technol. Inform. 2022, 289, 426–429.
- Mei, A.W.S.; Hong, P.L.; Keikhosrokiani, P.; Xin, C.H.; Ying, T.X.; Samat, N. A GIS-based Mobile Application to Improve Tourism Experience: A Case Study of Terengganu, Malaysia. In Proceedings of the 2021 International Congress of Advanced Technology and Engineering (ICOTEN), Taiz, Yemen, 4–5 July 2021; pp. 1–10.
- Aletdinova, A.A. Popular Mobile Applications for Crop Production. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 666, p. 032036.
- Monzon, J.P.; Calviño, P.A.; Sadras, V.O.; Zubiaurre, J.B.; Andrade, F.H. Precision agriculture based on crop physiological principles improves whole-farm yield and profit: A case study. Eur. J. Agron. 2018, 99, 62–71.
- Neupane, A.; Bulbul, I.; Wang, Z.; Lehman, R.M.; Nafziger, E.; Marzano, S.Y.L. Long term crop rotation effect on subsequent soybean yield explained by soil and root-associated microbiomes and soil health indicators. Sci. Rep. 2021, 11, 9200.
- Nesarajan, D.; Kunalan, L.; Logeswaran, M.; Kasthuriarachchi, S.; Lungalage, D. Coconut disease prediction system using image processing and deep learning techniques. In Proceedings of the 2020 IEEE 4th International Conference on Image Processing, Applications and Systems (IPAS), Genova, Italy, 9–11 December 2020; pp. 212–217.
- Thar, S.P.; Ramilan, T.; Farquharson, R.J.; Pang, A.; Chen, D. An empirical analysis of the use of agricultural mobile applications among smallholder farmers in Myanmar. Electron. J. Inf. 2021, 87, e12159.
- Sivagnanasundaram, J.; Ginige, A.; Goonetillake, J. Farmers as sensors: A crowdsensing platform to generate agricultural pest incidence reports. In Proceedings of the 2019 International Conference on Internet of Things Research and Practice (iCIOTRP), Sydney, Australia, 24–26 November 2019; pp. 13–18.
- Su, W.H. Advanced machine learning in point spectroscopy, RGB-and hyperspectral-imaging for automatic discriminations of crops and weeds: A review. Smart Cities 2020, 3, 767–792.
- Yang, X.F.; Kong, C.H. Interference of allelopathic rice with paddy weeds at the root level. Plant Biol. 2017, 19, 584–591.
- Nelson, S.C.; Corcoja, I.; Pethybridge, S.J. Cluster: A New Application for Spatial Analysis of Pixelated Data for Epiphytotics. Phytopathology 2017, 107, 1556–1566.
- Rossel, R.V.; Behrens, T.; Ben-Dor, E.; Brown, D.J.; Demattê, J.A.M.; Shepherd, K.D.; Shi, Z.; Stenberg, B.; Stevens, A.; Adamchuk, V.; et al. A global spectral library to characterize the world’s soil. Earth-Sci. Rev. 2016, 155, 198–230.
- Chen, S.S.; Fang, L.G.; Liu, Q.H.; Chen, L.F.; Tong, Q.X. The design and development of spectral library of featured crops of South China. In Proceedings of the 2005 IEEE International Geoscience and Remote Sensing Symposium, IGARSS’05, Seoul, Korea, 29 July 2005; Volume 2, p. 4.
- Lau, A.M.S.; Hashim, M. The design and building of spectral library of tropical rain forest in Malaysia. In Proceedings of the 28th Asian Conference on Remote Sensing, Kuala Lumpur, Malaysia, 12–16 November 2007; Asian Association on Remote Sensing: Tokyo, Japan; Volume 2, pp. 1150–1157.
- Jusoff, K.; Yusoff, M.M.; Ali, N.H.M. Spectral signatures of leaf fall diseases in Hevea brasiliensis using a handheld spectroradiometer. Mod. Appl. Sci. 2010, 4, 78–84.
- Ponnamperuma Arachchi, J.; Bandara, D.M.B.N.; Perera, S.P.M.G.N.H.; Nilakshi, S.V.; Nugaliyadde, L.; Sisira Kumara, W.A.G. An e-pest surveillance and advisory system to empower farmers in managing rice pests and diseases in Sri Lanka. In Proceedings of the International Research Conference on Smart Computing and Systems Engineering-SCSE, Department of Industrial Management, Faculty of Science, University of Kelaniya, Colombo, Sri Lanka, 29 March 2018.
- Mohapatra, S.D.; Tripathi, R.; Acharya, P.; Shahid, M.; Raghu, S.; Guru, P.K.; Dash, S.K. NRRI’riceXpert’APP: Taking rice technologies in the doorstep of farmers. In Proceedings of the Souvenir: 3rd ARRW International Symposium, Cuttack, India, 6–9 February 2018.
- Mostafa, S.A.; Hazeem, A.A.; Khaleefahand, S.H.; Mustapha, A.; Darman, R. A Collaborative Multi-agent System for Oil Palm Pests and Diseases Global Situation Awareness. In Proceedings of the Future Technologies Conference, Vancouver, BC, Canada, 13–14 November 2018; Springer: Cham, Switzerland, 2018; pp. 763–775.
- Rahim, S.E.; Supli, A.A.; Damiri, N. Developing a land suitability evaluation tool in mobile android application for rubber, cocoa and oil palm. J. Int. Soc. Southeast Asian Agric. Sci. 2016, 22, 80–90.
- De, A.; Singh, S.P. Analysis of fuzzy applications in the agri-supply chain: A literature review. J. Clean. Prod. 2021, 283, 124577.
- Yusof, M.M.; Rosli, N.F.; Othman, M.; Mohamed, R.; Abdullah, M.H.A. M-DCocoa: M-Agriculture Expert System for Diagnosing Cocoa Plant Diseases. In Proceedings of the International Conference on Soft Computing and Data Mining, Johor, Malaysia, 6–7 February 2018; Springer: Cham, Switzerland, 2018; pp. 363–371.
- Rachman, T.; Napitupulu, D. User acceptance analysis of potato expert system application based on TAM approach. Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 8, 185–191.
- Johannes, A.; Picon, A.; Alvarez-Gila, A.; Echazarra, J.; Rodriguez-Vaamonde, S.; Navajas, A.D.; Ortiz-Barredo, A. Automatic plant disease diagnosis using mobile capture devices, applied on a wheat use case. Comput. Electron. Agric. 2017, 138, 200–209.
- Ramcharan, A.; Baranowski, K.; McCloskey, P.; Ahmed, B.; Legg, J.; Hughes, D.P. Deep learning for image-based cassava disease detection. Front. Plant Sci. 2017, 8, 1852.
- Walls, J.T., III; Caciagli, P.; Tooker, J.F.; Russo, J.M.; Rajotte, E.G.; Rosa, C. Modeling the decision process for barley yellow dwarf management. Comput. Electron. Agric. 2016, 127, 775–786.
- Shrimali, S. PlantifyAI: A Novel Convolutional Neural Network Based Mobile Application for Efficient Crop Disease Detection and Treatment. Procedia Comput. Sci. 2021, 191, 469–474.
- Chen, C.J.; Huang, Y.Y.; Li, Y.S.; Chang, C.Y.; Huang, Y.M. An AIoT based smart agricultural system for pests detection. IEEE Access 2020, 8, 180750–180761.
- Roslin, N.A.; Che’Ya, N.N.; Sulaiman, N.; Nor Alahyadi, L.A.; Ismail, M.R. Mobile Application Development for Spectral Signature of Weed Species in Rice Farming. Pertanika J. Sci. Technol. 2021, 29, 2241–2259.
- Singh, N.K.; Dutta, A.; Puccetti, G.; Croll, D. Tackling microbial threats in agriculture with integrative imaging and computational approaches. Comput. Struct. Biotechnol. J. 2021, 19, 372–383.
- Triono, J.; Tristono, T. Expert System Identification of Pest and Diseases of Rice using Html5. Int. J. Adv. Res. Comput. Sci. Softw. Eng. 2016, 7, 60–63.
- Morco, R.C.; Calanda, F.B.; Bonilla, J.A.; Corpuz, M.J.S.; Avestro, J.E.; Angeles, J.M. E-Rice: An Expert System using Rule-Based Algorithm to Detect, Diagnose, and Prescribe Control Options for Rice Plant Diseases in the Philippines. In Proceedings of the 2017 International Conference on Computer Science and Artificial Intelligence, Jakarta, Indonesia, 5–7 December 2017; pp. 49–54.
- Adama, A.; Ee, K.P.; Sahari, N.; Tida, A.; Shang, C.Y.; Tawie, K.M.; Mohamad, H. Dr. LADA: Diagnosing black pepper pest and diseases with decision tree. Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 8, 1584–1590.
- Nasir, H.; Aris, A.N.; Lajis, A.; Kadir, K.; Safie, S.I. Development of Android Application for Pest Infestation Early Warning System. In Proceedings of the 2018 IEEE 5th International Conference on Smart Instrumentation, Measurement and Application (ICSIMA), Songkhla, Thailand, 28–30 November 2018; pp. 1–5.
- Novas, N.; Alvarez-Bermejo, J.A.; Valenzuela, J.L.; Gázquez, J.A.; Manzano-Agugliaro, F. Development of a smartphone application for assessment of chilling injuries in zucchini. Biosyst. Eng. 2019, 181, 114–127.
- Picon, A.; Alvarez-Gila, A.; Seitz, M.; Ortiz-Barredo, A.; Echazarra, J.; Johannes, A. Deep convolutional neural networks for mobile capture device-based crop disease classification in the wild. Comput. Electron. Agric. 2019, 161, 280–290.
- Valdez-Morones, T.; Pérez-Espinosa, H.; Avila-George, H.; Oblitas, J.; Castro, W. An Android App for detecting damage on tobacco (Nicotiana tabacum L.) leaves caused by blue mold (Penospora tabacina Adam). In Proceedings of the 2018 7th International Conference on Software Process Improvement (CIMPS), Guadalajara, México, 17–19 October 2018; pp. 125–129.
- Ranjith; Anas, S.; Badhusha, I.; Zaheema, O.T.; Faseela, K.; Shelly, M. Cloud based automated irrigation and plant leaf disease detection system using an android application. In Proceedings of the 2017 International conference of Electronics, Communication and Aerospace Technology (ICECA), Coimbatore, India, 20–22 April 2017; Volume 2, pp. 211–214.
- Tan, D.S.; Leong, R.N.; Laguna, A.F.; Ngo, C.A.; Lao, A.; Amalin, D.M.; Alvindia, D.G. AuToDiDAC: Automated tool for disease detection and assessment for cacao black pod rot. Crop Prot. 2018, 103, 98–102.
- Pérez-Castro, A.; Sánchez-Molina, J.A.; Castilla, M.; Sánchez-Moreno, J.; Moreno-Úbeda, J.C.; Magán, J.J. cFertigUAL: A fertigation management app for greenhouse vegetable crops. Agric. Water Manag. 2017, 183, 186–193.
- Katsaros, A.; Keramopoulos, E. FarmAR, a farmer’s augmented reality application based on semantic web. In Proceedings of the 2017 South Eastern European Design Automation, Computer Engineering, Computer Networks and Social Media Conference (SEEDA-CECNSM), Kastoria, Greece, 23–25 September 2017; pp. 1–6.
- Katamba, R.; Mutebi, B. Jaguza livestock app, the app transforming livestock production and strengthening food security. In Proceedings of the 2017 IST-Africa Week Conference (IST-Africa), Windhoek, Namibia, 31 May–2 June 2017; pp. 1–12.
- Machado, B.B.; Orue, J.P.; Arruda, M.S.; Santos, C.V.; Sarath, D.S.; Goncalves, W.N.; Rodrigues, J.F., Jr. BioLeaf: A professional mobile application to measure foliar damage caused by insect herbivory. Comput. Electron. Agric. 2016, 129, 44–55.
- Simorangkir, G.D.; Sarwoko, E.A.; Sasongko, P.S.; Endah, S.N. Usability Testing of Corn Diseases and Pests Detection on a Mobile Application. In Proceedings of the 2018 2nd International Conference on Informatics and Computational Sciences (ICICoS), Semarang, Indonesia, 30–31 October 2018; pp. 1–6.
- Petrellis, N. Plant disease diagnosis for smart phone applications with extensible set of diseases. Appl. Sci. 2019, 9, 1952.
- Rice Doctor—Apps on Google Play. May 2019. Available online: https://play.google.com/store/apps/details?id=com.lucidcentral.mobile.ricedoctor&hl=en (accessed on 9 January 2021).
- Miao, Z.; Yu, X.; Li, N.; He, C.; Sun, T. Weed Detection Based on the Fusion of Multiple Image Processing Algorithms. In Proceedings of the 2021 40th Chinese Control Conference (CCC), Shanghai, China, 26–28 July 2021; pp. 4217–4222.
- Mendes, J.; Pinho, T.M.; dos Santos, F.N.; Sousa, J.J.; Peres, E.; Boaventura-Cunha, J.; Cunha, M.; Morais, R. Smartphone applications targeting precision agriculture practices—A systematic review. Agronomy 2020, 10, 855.
- Ouhami, M.; Hafiane, A.; Es-Saady, Y.; El Hajji, M.; Canals, R. Computer vision, IoT and data fusion for crop disease detection using machine learning: A survey and ongoing research. Remote Sens. 2021, 13, 2486.
- Mrisho, L.M.; Mbilinyi, N.A.; Ndalahwa, M.; Ramcharan, A.M.; Kehs, A.K.; McCloskey, P.C.; Murithi, H.; Hughes, D.P.; Legg, J.P. Accuracy of a smartphone-based object detection model, PlantVillage Nuru, in identifying the foliar symptoms of the viral diseases of cassava–CMD and CBSD. Front. Plant Sci. 2020, 1964.
- Mutembesa, D.; Omongo, C.; Mwebaze, E. Crowdsourcing real-time viral disease and pest information: A case of nation-wide cassava disease surveillance in a developing country. In Proceedings of the Sixth AAAI Conference on Human Computation and Crowdsourcing, Zürich, Switzerland, 5–8 July 2018.
- Vasavi, P.; Punitha, A.; Narayana Rao, T.V. Crop leaf disease detection and classification using machine learning and deep learning algorithms by visual symptoms: A review. Int. J. Electr. Comput. Eng. 2022, 12, 2079–2086.
- Majid, K.; Herdiyeni, Y.; Rauf, A. I-PEDIA: Mobile application for paddy disease identification using fuzzy entropy and probabilistic neural network. In Proceedings of the 2013 International Conference on Advanced Computer Science and Information Systems (ICACSIS), Bali, Indonesia, 28–29 September 2013; pp. 403–406.
- Akbar, S. Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications; Springer: Cham, Switzerland, 2020.
- Towers, P.C.; Poblete-Echeverría, C. Effect of the Illumination Angle on NDVI Data Composed of Mixed Surface Values Obtained over Vertical-Shoot-Positioned Vineyards. Remote Sens. 2021, 13, 855.
- Hall, A.; Lamb, D.W.; Holzapfel, B.; Louis, J. Optical remote sensing applications in viticulture—A Review. Aust. J. Grape Wine Res. 2002, 8, 36–47.
- Zhu, J.Y.; He, W.J.; Wang, H.Q.; Yao, J.M.; Qin, G.M.; Xu, C.Y.; Huang, T. The Response of Spectral Characteristics and Leaf Functional Traits of Euonymus Japonicas to Leaf Dustfall. Spectrosc. Spectr. Anal. 2020, 40, 1620–1625.
- Al Shehhi, M.R.; Gherboudj, I.; Ghedira, H. Detection of algal blooms over optically complex waters of the Arabian Gulf and Sea of Oman using MODIS fluorescence data. Int. J. Remote Sens. 2019, 40, 3751–3771.
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.; Pintado, M.M.; Vasconcelos, M.W. Preserving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26.
- Veettil, B.K.; Ward, R.D.; Lima, M.D.A.C.; Stankovic, M.; Hoai, P.N.; Quang, N.X. Opportunities for seagrass research derived from remote sensing: A review of current methods. Ecol. Indic. 2020, 117, 106560.
- Landi, M.; Agati, G.; Fini, A.; Guidi, L.; Sebastiani, F.; Tattini, M. Unveiling the shade nature of cyanic leaves: A view from the “blue absorbing side” of anthocyanins. Plant Cell Environ. 2021, 44, 1119–1129.
- Eberlein, J.; Davenport, B.; Nguyen, T.T.; Victorino, F.; Jhun, K.; van der Heide, V.; Kuleshov, M.; Ma’ayan, A.; Kedl, R.; Homann, D. Chemokine signatures of pathogen-specific T cells I: Effector T cells. J. Immunol. 2020, 205, 2169–2187.
- Furlanetto, R.H.; Nanni, M.R.; Mizuno, M.S.; Crusiol, L.G.T.; da Silva, C.R. Identification and classification of Asian soybean rust using leaf-based hyperspectral reflectance. Int. J. Remote Sens. 2021, 42, 4177–4198.
- Golob, A.; Kavčič, J.; Stibilj, V.; Gaberščik, A.; Vogel-Mikuš, K.; Germ, M. The effect of selenium and UV radiation on leaf traits and biomass production in Triticum aestivum L. Ecotoxicol. Environ. Saf. 2017, 136, 142–149.
- Zheng, Y.; Fan, C.; Liu, M.; Chen, Y.; Lu, Z.; Xu, N.; Huang, H.; Zeng, H.; Liu, S.; Cao, H.; et al. Overall quality control of the chemical and bioactive consistency of ShengMai Formula. J. Pharm. Biomed. Anal. 2020, 189, 113411.
- Teofilović, B.; Grujić-Letić, N.; Gligorić, E.; Rašković, A.; Igić, R.; Vastag, G.; Gadžurić, S. Experimental and Computational Evaluation of Extraction Procedure and Scavenging Capacity of Sweet Basil Extracts (Ocimum basilicum L.). Plant Foods Hum. Nutr. 2021, 76, 240–247.
- Arias, F.; Zambrano, M.; Broce, K.; Medina, C.; Pacheco, H.; Nunez, Y. Hyperspectral imaging for rice cultivation: Applications, methods and challenges. AIMS Agric. Food 2021, 6, 273–307.
- Hernández-Clemente, R.; Hornero, A.; Mottus, M.; Peñuelas, J.; González-Dugo, V.; Jiménez, J.C.; Suárez, L.; Alonso, L.; Zarco-Tejada, P.J. Early diagnosis of vegetation health from high-resolution hyperspectral and thermal imagery: Lessons learned from empirical relationships and radiative transfer modelling. Curr. For. Rep. 2019, 5, 169–183.
- Mahlein, A.-K.; Alisaac, E.; Al Masri, A.; Behmann, J.; Dehne, H.-W.; Oerke, E.-C. Comparison and combination of thermal, fluorescence, and hyperspectral imaging for monitoring fusarium head blight of wheat on spikelet scale. Sensors 2019, 19, 2281.
- Guo, Y.; Chen, S.; Wu, Z.; Wang, S.; Robin Bryant, C.; Senthilnath, J.; Cunha, M.; Fu, Y.H. Integrating Spectral and Textural Information for Monitoring the Growth of Pear Trees Using Optical Images from the UAV Platform. Remote Sens. 2021, 13, 1795.
- Keller, M. The Science of Grapevines; Academic Press: Cambridge, MA, USA, 2020.
- Mandi, S.S. Natural UV Radiation in Enhancing Survival Value and Quality of Plants; Springer: New Delhi, India, 2016.
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi. Microbiol. Spectr. 2017, 5, 5.1.14.
- Marın-Ortiz, J.C.; Gutierrez-Toro, N.; Botero-Ferna´ndez, V.; Hoyos- Carvajal, L.M. Linking physiological parameters with visible/ near-infrared leaf reflectance in the incubation period of vascular wilt disease. Saudi J. Biol. Sci. 2020, 27, 88–99.
- Soja-Woźniak, M.; Craig, S.E.; Kratzer, S.; Wojtasiewicz, B.; Darecki, M.; Jones, C.T. A novel statistical approach for ocean colour estimation of inherent optical properties and cyanobacteria abundance in optically complex waters. Remote Sens. 2017, 9, 343.
- Meng, R.; Lv, Z.; Yan, J.; Chen, G.; Zhao, F.; Zeng, L.; Xu, B. Development of Spectral Disease Indices for Southern Corn Rust Detection and Severity Classification. Remote Sens. 2020, 12, 3233.
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844.
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced methods of plant disease detection. A review. Agron. Sustain. Dev. 2015, 35, 1–25.
- Al-lami, A.K.; Abbood, R.A.; Al Maliki, A.A.; Al-Ansari, N. Using vegetation indices for monitoring the spread of Nile Rose plant in the Tigris River within Wasit province, Iraq. Remote Sens. Appl. Soc. Environ. 2021, 22, 100471.
- Thamaga, K.H.; Dube, T. Testing two methods for mapping water hyacinth (Eichhornia crassipes) in the Greater Letaba river system, South Africa: Discrimination and mapping potential of the polar-orbiting Sentinel-2 MSI and Landsat 8 OLI sensors. Int. J. Remote Sens. 2018, 39, 8041–8059.
- Bradly, B. Remote detection of invasive plants: A review of spectral, textural and phenological approaches. Biol. Invaions 2014, 16, 1411–1425.
- Paz-Kagan, T.; Silver, M.; Panov, N.; Karnieli, A. Multispectral approach for identifying invasive plant species based on flowering phenology characteristics. Remote Sens. 2019, 11, 953.
- Dube, T.; Mutanga, O.; Sibanda, M.; Bangamwabo, V.; Shoko, C. Evaluation the performance of the newly-launched Landsat 8 sensor in detecting and mapping the spatial confguration of water hyacinth (Eichhornia crassipes) in inland lakes, Zimbabwe. Phys. Chem. Earth Parts A/B/C 2017, 100, 101–111.
- Chander, S.; Pompapathi, V.; Gujrati, A.; Singh, R.P.; Chaplot, N.; Patel, U.D. Growth of invasive aquatic macrophytes over Tapi river. In Proceedings of the International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, Volume XLII- 5, 2018 ISPRS TC V Mid-term Symposium “Geospatial Technology—Pixel to People”, Dehradun, India, 20–23 November 2018.
