Magnetite nanoparticles with different surface coverages are of great interest for many applications due to their intrinsic magnetic properties, nanometer size, and definite surface morphology. Magnetite nanoparticles are widely used for different medical-biological applications while their usage in optics is not as widespread. In recent years, nanomagnetite suspensions, so-called magnetic ferrofluids, are applied in optics due to their magneto-optical properties.
- magnetite nanoparticles
- magnetic ferrofluids
- synthesis
- application
- optical devices
Magnetite Nanoparticles
Magnetite nanoparticles with different surface coverages are of great interest for many applications due to their intrinsic magnetic properties, nanometer size, and definite surface morphology. Magnetite nanoparticles are widely used for different medical-biological applications while their usage in optics is not as widespread. In recent years, nanomagnetite suspensions, so-called magnetic ferrofluids, are applied in optics due to their magneto-optical properties.
Magnetic nanoparticles Magnetic ferrofluids synthesis application
- Introduction
Nanotechnology is a field of fundamental and applied science dealing with methods of research, methods of production, and the use of materials with defined atomic structures through the controlled manipulation of individual atoms and molecules. Nanotechnology is focused on the creation, investigation, and application of new types of materials, called “nanomaterials”, of which the size of at least one dimension is from 1 to 100 nm. Nanomaterials find their application in the broadest fields, including the chemical industry [1,2,3], agriculture [4,5], engineering [6], pharmaceutics [7], sustainable energy [8], medicine [9,10,11], etc.
In the last two decades, nanomaterials such as magnetic nanoparticles (MNPs) were widely utilized for a huge number of applications due to their specific magnetic properties, high surface area, and unique dimensions. Among them are NPs of different magnetic structures: nanodots [12], nanorods [13], nanowires [14,15], nanotubes [16], core–shell nanoparticles [17], etc. The chemical composition of MNPs also varies widely, including pure metals, alloys, metal oxides, and doped NPs. MNPs could be further coated and functionalized according to the needs of their consumers. Here, we highlight magnetite nanoparticles among a giant family of MNPs.
Considerable efforts are devoted to the creation of synthetic MNPs and to various strategies for their synthesis. In general, two main approaches to nanomagnetite synthesis exist: top-down and bottom-up. Top-down approaches consist of breaking up large pieces of the initial material to produce nanoparticles and include the use of physical methods, such as ball milling, electron beam lithography, etc. Bottom-up approaches imply the creation of nanoparticles from molecules and include the use of both chemical and biological methods. Attaining NPs in the range of nanometers using physical methods is difficult; thus, more efforts should be directed toward creating and improving chemical and biological methods of synthesis in order to obtain MNPs with desirable sizes, shapes, crystallinities, and compositions. However, a question remains to be answered: what method is most suitable for MNP production? Every method has its limitation, so the choice of synthesis method depends on the desired application of the MNP.
As MNPs are easily oxidizable by oxygen in the environment and agglomerate due to their large specific surface area, developing specific functional coatings (surface functionalization) that would protect the MNPs and further decorate MNPs with therapeutic agents, fluorescent labels, etc. is necessary.
MNPs are thought to be promising for a wide range of applications because of their unique characteristics, including their high saturation magnetization, which makes them easily operated by the magnetic field, and their low toxicity. They are broadly applied and have potential for biomedical applications, such as magnetic drug targeting [18,19,20], DNA/RNA purification [21,22,23], magnetofection [24,25], hyperthermia [26,27,28,29], MRI imaging [30,31], cell separation [32], etc. MNPs also could be utilized for the creation of ferrofluids (FF)—colloidal suspension of NPs in polar or non-polar liquid carriers. The main distinctive features of FFs are the fluidities of liquid material and the magnetism of MNPs. FFs have unique magnetic properties in the presence of magnetic fields such as optical anisotropy, birefringence, and field-dependent transmission. These unique properties of FFs make them a promising platform for novel optoelectronic devices, such as optical fiber sensors, optical gratings, organic light-emitting diods, agents for the enhancing of MRI images, photonic materials, etc. The use of FFs in optics and nanophotonics faces numerous challenges; thus, investigations in this field are necessary.
. Structure and Properties of Magnetite
Magnetite, Fe3O4, an iron oxide, has an inverse spinel structure (Figure 1). The details of its structure were first established in 1915 by W.H. Bragg [33], who provided one of the first mineral structures determined using the X-ray diffraction method. Magnetite has a cubic unit cell with a lattice constant of a = 0.839 nm and is an iron oxide of mixed valences Fe3+ and Fe2+ within the stoichiometry Fe2+/Fe3+ = 0.5. The main feature of the spinel crystal lattice is the presence of two crystallographic positions of iron ions: Fe2+ and half of the Fe3+ ions occupying octahedral sites (surrounded by six oxygen O2−), and the other half of the Fe3+ ions occupying tetrahedral sites (surrounded by four oxygen O2−). Magnetite is frequently non-stoichiometric, resulting in a cation-deficient Fe3+ layer. Fe2+ could also be fully or partly replaced by other divalent ions (Mg2+, Co2+, Ni2+, etc.), which leads to changes in the lattice constant.
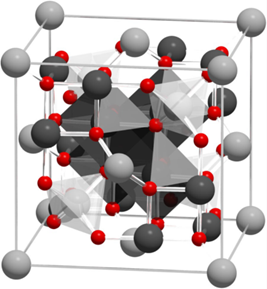
Figure 1. Visualization of the magnetite unit cell identified using octahedral Fe2,5+ (dark grey), tetrahedral Fe2+ (light grey), and oxygen (red). The local site symmetries are shown by the octahedral and tetrahedral shapes around fully coordinated Fe sites within the unit cell. The different bond angles between the Fe sites lead to dominant antiferromagnetic coupling between the tetrahedral and octahedral sites, giving a bulk ferrimagnetic order (adapted from [34] with permission from Springer Nature).
The magnetic properties of magnetite are determined by its crystal structure. Namely, the ordering of magnetic spin moments of iron cations in the crystal lattice occurs through a special interaction between electrons of the 3d shell of neighboring cations. The ordering of magnetic spin moments leads to the overall magnetic ordering in magnetite. Magnetite possesses magnetocrystalline anisotropy, a special case of magnetic anisotropy that is related to spin-orbit coupling. Magnetocrystalline anisotropy depends on the magnetite crystal structure, shape, and chemical composition and plays a critical role in the magnetic ordering of magnetite. The unique magnetic and structural properties of magnetite, in comparison, for example, with ferrites, are determined also by the presence of so-called hopping electrons. Briefly, 3d electron transfer occurs in the Fe2+–Fe+3 octahedral cation pair, which could be described in short as Fe2+(3d6) ↔ Fe3+(3d5). The concentration of hopping electrons in magnetite is high in comparison with spinel ferrites, which have similar lattice structures, and thus makes a significant contribution to the formation of its magnetic properties. To conclude, magnetite at room temperature is ferrimagnetic with a Curie temperature of 580 °C (Table 1), which has a rather high saturation magnetization value of 92 A·m2/kg.
Table 1. Properties of magnetite.
|
Properties |
Magnetite |
|
Molecular formula |
Fe3O4 |
|
Crystal structure |
Cubic |
|
Density (gm/cm3 ) |
5.18 |
|
Melting point (°C) |
1583–1597 |
|
Boiling point (°C) |
2623 |
|
Color |
black |
|
Hardness |
5.5 |
|
Type of magnetism |
ferrimagnetic |
|
Curie temperature (K) |
580 |
|
Ms at 300 K (A·m2/kg) |
92–100 |
|
Magnetism (nanoparticles) |
Superparamagnetic |
|
Lustre |
Metallic |
|
Diaphaneity |
Opaque |
|
Crystal System |
Isometric |
|
Birefringence |
Isotropic minerals have no birefringence |
|
Refractive Index values |
n = 2.42 |
References
- Piracha, S.; Saleem, S.; Momil, G.; Anjum, A.; Yaseen, Z. Nanoparticle: Role in Chemical Industries, Potential Sources and Chemical Catalysis Applications. Int. J. Chem. Mater. Sci. 2020, 4, 40–45. https://doi.org/10.36348/sijcms.2021.v04i04.006.
- Subhan, A.; Choudhury, K.P.; Neogi, N. Advances with Molecular Nanomaterials in Industrial Manufacturing Applications. J. Nanomanufacturing 2021, 1, 75–97. https://doi.org/10.3390/nanomanufacturing1020008.
- Pawar, S.; Duadi, H.; Fleger, Y.; Fixler, D. Carbon Dots-Based Logic Gates. Nanomaterials 2021, 11, 232. https://doi.org/10.3390/nano11010232.
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Microbiol. 2017, 8, 124–127. https://doi.org/10.3389/fmicb.2017.01014.
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Alam Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Total. Environ. 2020, 721, 137778. https://doi.org/10.1016/j.scitotenv.2020.137778.
- Barnoy, E.A.; Popovtzer, R.; Fixler, D. Fluorescence for biological logic gates. Biophotonics 2020, 13, e202000158. https://doi.org/10.1002/jbio.202000158.
- Finke, J.H.; Juhnke, M.; Kwade, A.; Bunjes, H.; Cornier, J.; Owen, A.; Van de Voorde, M. Overview of techniques and description of established processes. In Pharmaceutical Nanotechnology: Innovation and Production, 1st. ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; p. 772.
- Serrano, E.; Rus, G.; García-Martínez, J. Nanotechnology for sustainable energy. Sustain. Energy Rev. 2009, 13, 2373–2384. https://doi.org/10.1016/j.rser.2009.06.003.
- Mabrouk, M.; Das, D.; Salem, Z.; Beherei, H. Nanomaterials for Biomedical Applications: Production, Characterisations, Recent Trends and Difficulties. Molecules 2021, 26, 1077. https://doi.org/10.3390/molecules26041077.
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Surf. Sci. Adv. 2021, 6, 100163. https://doi.org/10.1016/j.apsadv.2021.100163.
- Aflori, M. Smart Nanomaterials for Biomedical Applications—A Review. Nanomaterials 2021, 11, 396. https://doi.org/10.3390/nano11020396.
- Arcudi, F.; Đorđević, L.; Prato, M. Design, Synthesis, and Functionalization Strategies of Tailored Carbon Nanodots. Chem. Res. 2019, 52, 2070–2079. https://doi.org/10.1021/acs.accounts.9b00249.
- An, L.; Wang, Y.; Tian, Q.; Yang, S. Small Gold Nanorods: Recent Advances in Synthesis, Biological Imaging, and Cancer Therapy. Materials 2017, 10, 1372. https://doi.org/10.3390/ma10121372.
- Parente, M.; Van Helvert, M.; Hamans, R.F.; Verbroekken, R.; Sinha, R.; Bieberle-Hütter, A.; Baldi, A. Simple and Fast High-Yield Synthesis of Silver Nanowires. Nano Lett. 2020, 20, 5759–5764. https://doi.org/10.1021/acs.nanolett.0c01565.
- Vorobjova, A.; Tishkevich, D.; Shimanovich, D.; Zubar, T.; Astapovich, K.; Kozlovskiy, A.; Zdorovets, M.; Zhaludkevich, A.; Lyakhov, D.; Michels, D.; et al. The influence of the synthesis conditions on the magnetic behaviour of the densely packed arrays of Ni nanowires in porous anodic alumina membranes. RSC Adv. 2021, 11, 3952–3962. https://doi.org/10.1039/d0ra07529a.
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. https://doi.org/10.1007/s42823-019-00068-2.
- Chen, H.; Zhang, L.; Li, M.; Xie, G. Synthesis of Core–Shell Micro/Nanoparticles and Their Tribological Application: A Review. Materials 2020, 13, 4590. https://doi.org/10.3390/ma13204590.
- Kianfar, E. Magnetic Nanoparticles in Targeted Drug Delivery: A Review. Supercond. Nov. Magn. 2021, 34, 1709–1735. https://doi.org/10.1007/s10948-021-05932-9.
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Chem. 2018, 6, 619. https://doi.org/10.3389/fchem.2018.00619.
- Liu, Y.-L.; Chen, D.; Shang, P.; Yin, D.-C. A review of magnet systems for targeted drug delivery. Control. Release 2019, 302, 90–104. https://doi.org/10.1016/j.jconrel.2019.03.031.
- Chacón-Torres, J.C.; Reinoso, C.; Navas-León, D.G.; Briceño, S.; González, G. Optimized and scalable synthesis of magnetic nanoparticles for RNA extraction in response to developing countries’ needs in the detection and control of SARS-CoV-2. Rep. 2020, 10, 19004. https://doi.org/10.1038/s41598-020-75798-9.
- Khatami, F.; Najafi, F.; Yari, F.; Khavari-Nejad, R.A. Magnetic nanoparticles: A promising component in RNA extraction process. Biol. Sci. 2017, 7, 47–52. https://doi.org/10.22059/PBS.2018.244649.1285.
- Tang, C.; He, Z.; Liu, H.; Xu, Y.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y.; et al. Application of magnetic nanoparticles in nucleic acid detection. Nanobiotechnol. 2020, 18, 1–19. https://doi.org/10.1186/s12951-020-00613-6.
- Grzeskowiak, B.; Sánchez-Antequera, Y.; Hammerschmid, E.; Döblinger, M.; Eberbeck, D.; Woźniak, A.; Słomski, R.; Plank, C.; Mykhaylyk, O. Nanomagnetic Activation as a Way to Control the Efficacy of Nucleic Acid Delivery. Res. 2015, 32, 103–121. https://doi.org/10.1007/s11095-014-1448-6.
- Zuvin, M.; Kuruoglu, E.; Kaya, V.O.; Unal, O.; Kutlu, O.; Acar, H.Y.; Gozuacik, D.; Koşar, A. Magnetofection of Green Fluorescent Protein Encoding DNA-Bearing Polyethyleneimine-Coated Superparamagnetic Iron Oxide Nanoparticles to Human Breast Cancer Cells. ACS Omega 2019, 4, 12366–12374. https://doi.org/10.1021/acsomega.9b01000.
- Kalubowilage, M.; Janik, K.; Bossmann, S.H. Magnetic Nanomaterials for Magnetically-Aided Drug Delivery and Hyperthermia. Sci. 2019, 9, 2927. https://doi.org/10.3390/app9142927.
- Rajan, A.; Sahu, N.K. Review on magnetic nanoparticle-mediated hyperthermia for cancer therapy. Nanopart. Res. 2020, 22, 1–25. https://doi.org/10.1007/s11051-020-05045-9.
- Chang, D.; Lim, M.; Goos, J.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Pharmacol. 2018, 9, 831. https://doi.org/10.3389/fphar.2018.00831.
- Fatima, H.; Charinpanitkul, T.; Kim, K.-S. Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy. Nanomaterials 2021, 11, 1203. https://doi.org/10.3390/nano11051203.
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Curr. Chem. 2020, 378, 1–43. https://doi.org/10.1007/s41061-020-00302-w.
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Ruiz-Cabello, J.; Herranz, F.; Pellico, J. Iron Oxide Nanoparticles: An Alternative for Positive Contrast in Magnetic Resonance Imaging. Inorganics 2020, 8, 28. https://doi.org/10.3390/inorganics8040028.
- Haghighi, A.H.; Khorasani, M.T.; Faghih, Z.; Farjadian, F. Effects of different quantities of antibody conjugated with magnetic nanoparticles on cell separation efficiency. Heliyon 2020, 6, e03677. https://doi.org/10.1016/j.heliyon.2020.e03677.
- Bragg, W.H. The Structure of Magnetite and the Spinels. Nature 1915, 95, 561–561. https://doi.org/10.1038/095561a0.
This entry is adapted from the peer-reviewed paper 10.3390/ma15072601
