After being absorbed by the intestinal epithelium, flavonoids undergo extensive biotransformation into conjugated products, namely glucuronides, sulphates, and methylated derivatives, first in the intestine and then in the liver, where they are secreted into bile
[16][17]. Thus, the bioavailability and the subsequent cell and tissue accumulation of the different flavonoids essentially depend on the multidrug-resistance-associated proteins (MRP-1 and MRP-2), ubiquitously expressed as ATP-dependent efflux transporters. The actual flux of a flavonoid from the gut lumen to the blood stream and the various organs depends on the tissue distribution of MRP-1 and MRP-2 as well as on their substrate’s affinity. This metabolic pathway is called phase III metabolism. However, it appears that certain phase II metabolic derivates of flavonoids can act as competitive substrates of the MRP-mediated membrane transporters and the potential use of flavonoids as a mean to overcome transporter-mediated chemotherapy resistance due to the frequent overexpression of MRP in several types of cancer is based on this property. The intestinal absorption of quercetin, for instance, is favored in the aglycone form, and its metabolism in the gut and liver appears to be relatively high, so that less than 2% of ingested quercetin is recovered on the plasma
[3]. Additionally, after oral administration of flavonoids, a significant amount can reach the colon and can interact with microbiota. Microbiota can, for instance, metabolize some flavonoids to smaller phenolic compounds with similar biological effects and improved bioavailability; however, on the other hand, it can also extensively metabolize flavonoids via the glucuronidase and sulfatase enzymes, cleaving the heterocycle break and producing inert polar compounds that are rapidly excreted without producing any biological effect
[13].
In addition, flavonoids have been reported to significantly inhibit the activity of the cytochrome P450 system, which can result in an increase of the half-life and concentration of many drugs, thus enhancing their toxicity and side effects
[18]. Flavonoids such as quercetin, ECG, EGCG, and sylibin have been shown to downregulate the cytochrome CYP3A4, which is the major cytochrome P450 isoenzyme in the intestine and is responsible for the metabolism of approximately 50% of all prescribed drugs, thus increasing the risk of potential toxicity, especially of drugs with a limited therapeutic window
[19]. Flavonoids can also interact with ATP-binding cassette (ABC) transporters, inhibiting them, which can increase the bioavailability of poorly available drugs, on the one hand, but it can also potentiate the toxicity of other ABC transporters substrates
[20]. Thus, flavonoid encapsulation in effective nano-carrier systems can not only improve their pharmacokinetics and therapeutic potential but also avoid enhancement of the toxicity and side effects of drugs that can concomitantly be administrated with these compounds
[13].
4. Cutaneous Delivery Systems of Flavonoids for Treatment of Skin Pathologies
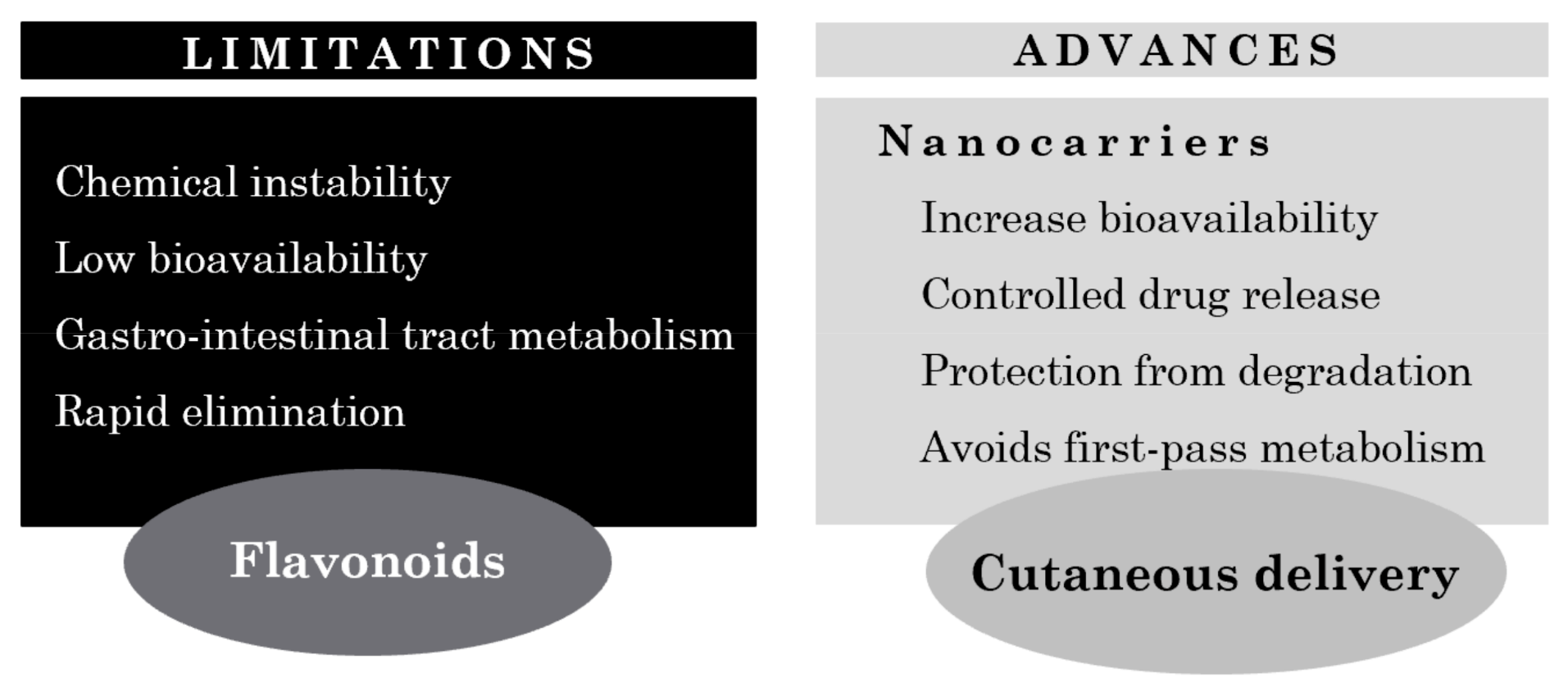
Cutaneous delivery of flavonoids is a powerful strategy to avoid systemic toxicity while restricting the therapeutic effects to the specific site of action. However, one of the major challenges that a topical delivery system faces is the ability to overcome the
SC barrier against foreign substances
[5]. In addition, most flavonoids are highly lipophilic compounds and their permeation across the
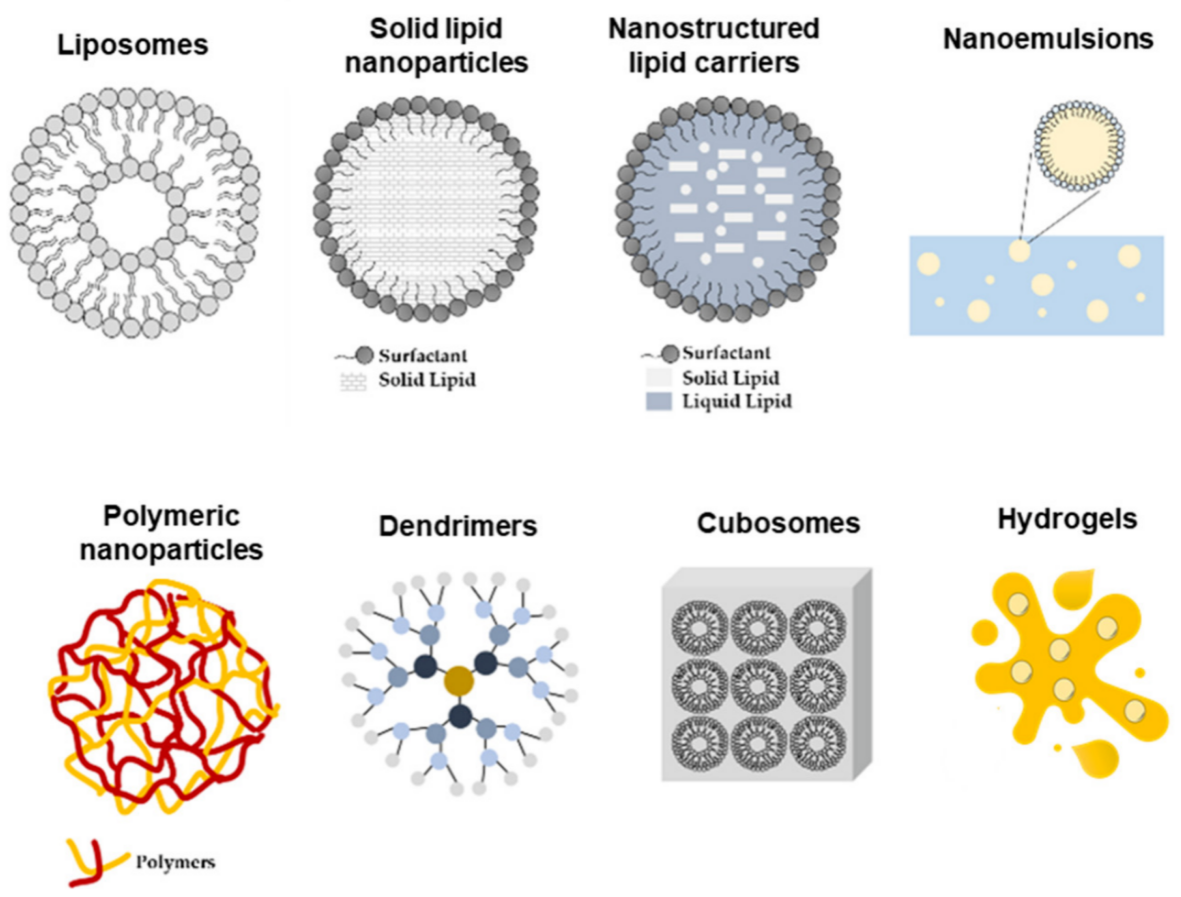
SC into viable skin layers is hindered by their affinity for SC components and the tendency to be retained in this layer. Thus, there has been a growing interest in the use of nanotechnology as a strategy for a more efficient flavonoid delivery to the human body (
Figure 3). Nano-delivery systems are in fact excellent tools to overcome the challenges associated not only with the cutaneous absorption of the drug per se but also with flavonoid pharmacology, including low solubility, short half-life, and poor bioavailability
[5][23].
Figure 3. Limitations and advances on cutaneous flavonoid delivery.
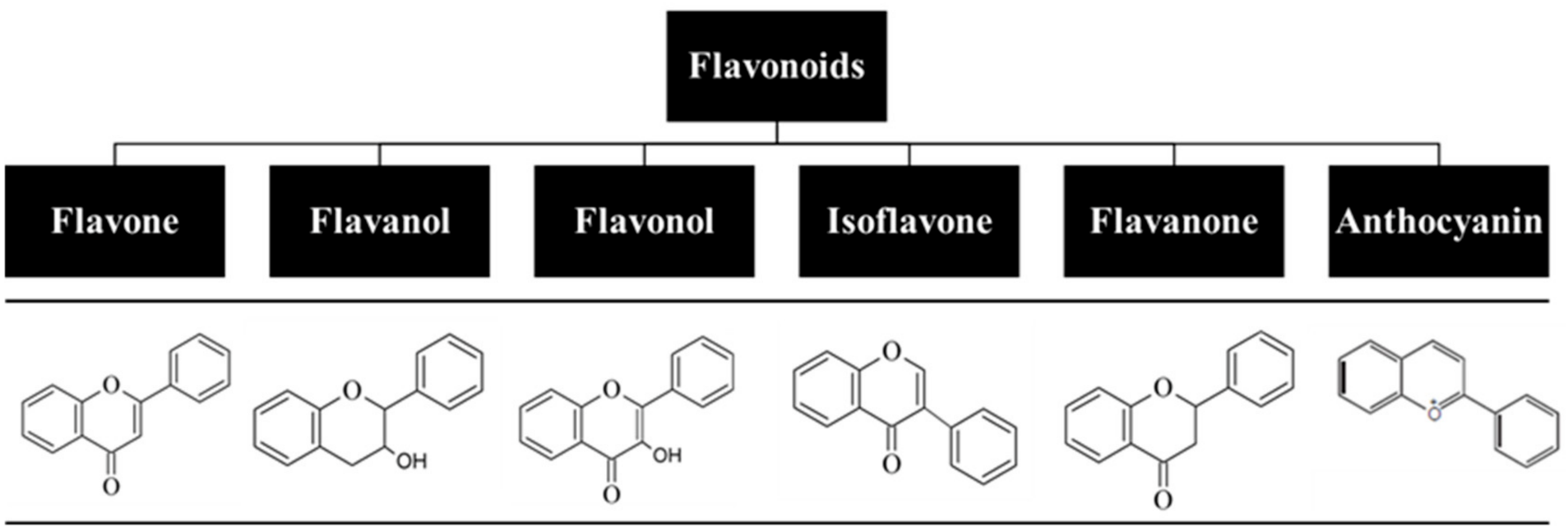
4.1. Examples of Nanocarriers Designed for Flavonol Cutaneous Delivery
Flavonols are O-glycosidic ketonic compounds with a sugar moiety at the 3-position that act as powerful antioxidants, protecting the skin from ROS formation. Compounds belonging to this family of flavonoids are quercetin, kaempferol, and myricetin, among others
[24].
Quercetin, one of the best studied and most common flavonoid found in nature, was shown to have poor permeability across excised human skin
[4]. For that, this flavonoid has been incorporated into different delivery systems, including nanoemulsions, nanocapsules, lipid nanoparticles, and microemulsions, to increase its solubility and skin permeability
[5]. Casagrande and colleagues incorporated quercetin into two different oil-in-water emulsions with a distinct lipid content in order to evaluate their potential application as a topical delivery system. The in vivo results demonstrated that these formulations were an effective vehicle for topical application of quercetin with the goal of controlling ultraviolet B (UVB)-induced skin damage
[4][25]. Based on these results, other studies were conducted to design novel delivery systems to increase quercetin effectiveness when topically applied. For example, quercetin was incorporated into a liquid, crystalline formulation and the influence of this vehicle in the antioxidant activity of this flavonoid was evaluated in vitro. The presence of a liquid, crystalline structure allowed for an easier diffusion through the skin and a considerable solubilizing capacity for both oil- and water-soluble compounds. Scalia and colleagues also demonstrated that the incorporation of quercetin in lipid microparticles improved its photostability and chemical stability as well as its biocompatibility
[26][27]. In another study, Tan and colleagues investigated the potential of using lecithin-chitosan nanoparticles as a topical delivery system for quercetin. Compared with quercetin in its free form, the quercetin-loaded nanoparticles displayed higher permeation ability and significant accumulation of quercetin in the skin, particularly in the epidermis. In addition, microstructure observations of the skin surface following administration showed that the interaction between constituents of the nanoparticles and the skin surface markedly changed the morphology of the
SC and disrupted the corneocyte layers, therefore facilitating permeation and accumulation of quercetin in the skin
[28].
Nan and colleagues evaluated the efficacy of topically applying quercetin-loaded chitosan nanoparticles against UVB radiation. The authors demonstrated that quercetin, if entrapped into chitosan nanoparticles, could be efficiently up taken by HaCaT cells (keratinocytes) and could easily permeate through the epidermis layer while displaying better stability and lower cytotoxicity. Moreover, they also found that quercetin-loaded nanoparticles could enhance the effects of this flavonoid when inhibiting the NF-kB/COX-2 signaling pathway as well as when ameliorating the skin edema caused by UVB radiation
[29].
Bose and Michniak-Kohn developed a solvent-free NLC formulation of quercetin using probe ultrasonication and evaluated the feasibility for topical delivery. Formulation factors such as the nature of the lipid (solid/combination of solid and liquid) in the SLN and NLC systems and the drug loading capacity were evaluated to produce the optimal formulation with an adequate physical stability. Overall, the NLC system showed the highest improvement in the topical delivery of quercetin, manifested by the amount of quercetin retained in full-thickness human skin compared with a control formulation with a similar composition and particle size in the micrometer range, thus demonstrating the feasibility of NLC systems for improved cutaneous delivery of this compound
[30].
Penetration enhancer containing vesicles (PEVs) are also known to be powerful enhancers for dermal delivery due to the presence of both phospholipids and penetration enhancers (PE), which provide a synergistic effect on skin permeation. PE increases the fluidity of the lipids of the SC, facilitating the delivery of drug-loaded vesicles and its subsequent diffusion through the skin
[5]. Hence, in 2011, Chessa and colleagues developed quercetin-loaded PEVs, formulated using four different hydrophilic PE, and characterized them by size, surface charge, loading capacity, and morphological and viscoelastic features. In addition, their penetration capability and distribution through pig skin were assessed to obtain the optimal formulation for the delivery of quercetin to the skin
[31]. In another study, performed by Caddeo and colleagues, quercetin-loaded phospholipid vesicles, in particular liposomes and PEVs, were developed in order to study their efficacy on 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin inflammation. In vivo results demonstrated that the vesicles, in particular PEVs, were capable of delivering the drug to the inflammation site, that is the dermis, inhibiting oxidative stress and leukocyte accumulation as well as stimulating the repair of skin damaged induced by TPA. Through this work, the authors highlighted the use of vesicular systems, particularly PEVs, as a delivery vehicle for flavonoids, with a therapeutic potential to treat inflammatory skin disorders
[32].
Kaempferol is another well-known flavonol with antioxidant, anti-inflammatory, anticancer, and antiallergic properties
[33]. In fact, Wang and colleagues reported that kaempferol inhibited the iNOS mRNA expression and prostaglandin E2 production in a dose-dependent manner, by inhibiting in part the NF-kB signaling pathway
[34]. Furthermore, Park et al. reported that kaempferol was also able to inhibit the activation of inflammatory NF-kB transcription factor via nuclear factor-inducing kinase (NIK)/IkB kinase (IKK) and MAPKs in aged rat kidney
[35]. Nonetheless, studies have shown that this flavonoid undergoes excessive first pass metabolism and, as a consequence, displays a bioavailability rate of only 2%, thus making it a good candidate for cutaneous application
[33]. Keeping that in mind, Yun Chao and colleagues developed submicron emulsions to be employed as a delivery system for the topical application of kaempferol. These submicron emulsion systems are oil-in-water dispersions with small droplet sizes in the range of 100–600 nm. In comparison with traditional drug delivery vehicles, they are easy to manufacture, are more thermodynamically stable, and exhibit enhanced drug solubilization as well as increased drug permeation rates. Additionally, submicron emulsions were demonstrated to be a potential vehicle for the transdermal and topical delivery of lipophilic and hydrophilic drugs. In the study, kaempferol-loaded submicron emulsions with different water/oil/surfactant/cosurfactant ratios were prepared, and different physiochemical properties (e.g., viscosity, droplet size, permeation rate, lag time, and deposition amount in skin) were determined in order to evaluate the effectiveness of this delivery system for the cutaneous application of kaempferol. Overall, the authors demonstrated that, based on the permeation parameters, including the increase in the cumulative amount of drug over 12 h and deposition in the skin, in addition to a shorter lag time, submicron emulsions may be a promising vehicle for cutaneous application of kaempferol
[33].
4.2. Examples of Nanocarriers Designed for Other Flavonoid Classes’ Topical Delivery
Isoflavones, are naturally occurring isoflavonoids mainly found in soybeans, soy foods, and legumes. They are non-steroidal compounds that act as phytoestrogens as they exert pseudohormonol activity by binding to estrogen receptors in mammals. The most common isoflavones are genistein and daidzein
[5]. Huang and colleagues assessed the potential topical delivery and dermal use of soy isoflavones genistein and daidzein, using α-terpineol and oleic acid as PE, both in vitro and in vivo. As demonstrated in vivo, there was an increase in the uptake of genistein an daidzein, with no toxic effects, and a decrease in the erythema. In vitro studies showed an inhibition of UVB-induced intracellular H
2O
2 production and the consequent protection of keratinocytes against UVB radiation, suggesting that a reduction in photodamage to the skin via the topical application of antioxidants could be an efficient way to enrich the endogenous cutaneous protection system
[34][36].
Apigenin is a hydrophobic, polyphenolic flavonoid known to possess antioxidant, antimicrobial, anti-inflammatory, antiviral, antidiabetic, and tumor inhibitory activities. In particular, this flavonoid was demonstrated to act as a chemo-preventive by inhibiting the enzyme CYP2C and by preventing the metabolism of many drugs and xenobiotics. Similar to the already mentioned flavonoids, the clinical potential of apigenin is suppressed by its poor aqueous solubility, low oral bioavailability, and rapid metabolism. Thus, the development of novel formulations is a necessary step to overcome these limitations and to improve apigenin delivery
[5]. On that matter, several formulations have been developed so far, including liposomes, nanocrystal gel formulations, and self-micro-emulsifying drug delivery systems. Munyendo and colleagues reported that the formulation of D-α-tocopherol acid and polyethylene glycol 1000 succinate (TPGS) stabilized the mixed micelles of apigenin and phospholipids, creating an effective drug delivery vehicle capable of enhancing the bioavailability of this flavonoid
[37]. Karthivashan and colleagues prepared “flavonosomes”, which are phytosomes loaded with multiple flavonoids, using phosphatidylcholine as a carrier and evaluated their in vitro pharmacokinetics and toxicity
[38]. Shen and co-workers evaluated a novel topical delivery system for apigenin by using soy lecithin-based ethosomes, demonstrating a higher skin targeting capacity and a significant reduction in COX-2 levels in mouse skin inflammation induced by UVB light
[39].
Luteolin is another promising flavonoid with potential antiarthritic activity. In addition, due to its lipophilicity, it can be used in topical formulations to treat psoriasis
[40]. Niosomes are non-ionic surfactant-based colloidal systems that have the ability to encapsulate both hydrophobic and hydrophilic drugs. Abidin and co-workers prepared luteolin-loaded niosomes using different non-ionic surfactants and characterized them for their in vitro and in vivo antiarthritic activity. The optimized formulation was later converted into gel using Carbopol as a gelling agent for enhanced transdermal luteolin delivery. The in vivo bioactive studies revealed that the niotransgel formulation of luteolin was able to provide good antiarthritic activity, with the results being comparable with standard diclofenac gel formulation
[41]. In another study, Shin and colleagues, established a nanoemulsion-based follicular delivery system, in which luteolin was incorporated into oil-in-water nanoemulsions. In vivo studies proved that these luteolin-loaded nanoemulsions possessed hair-growth promotion ability. In fact, when nanoemulsions are formed by the assembly of amphiphilic polymers at the oil/water (O/W) interface, they provide an efficient system for the encapsulation of poorly water-soluble substances, resulting in better bioavailability, accurate dosing, and minimal side effects
[42].
Catechins are a group of flavonoids that belong to the flavanol family and are present in high concentrations in a variety of plant-based fruits, vegetables, and beverages. Belonging to this family are catechin, epicatechin (EG), and EGCG. EGCG, in particular, has captured a lot of attention due to its broad spectrum of biological properties, including antioxidant, photoprotective, antiviral, and antibacterial as well as anticancer and neuroprotective effects. Nevertheless, its clinical use has been limited due to its poor systemic absorption and low bioavailability
[5]. With the goal to overcome this problem and to increase EGCG clinical applicability, Avadhani and co-workers developed nano-transfersomal formulations of EGCG for an efficient permeation into the SC and delivery into the skin
[43]. In addition, hyaluronic acid (HA) was also encapsulated in the transfersomes not only because it is widely distributed in connective tissues and is a main component of the extracellular matrix but also because it is a non-irritating biopolymer and antiaging agent with high biocompatibility, specific viscoelasticity, and hydration and lubrification properties. The optimized transfersomal formulation containing EGCG and HA displayed a high free radical scavenging effect while showing no cell toxicity. In addition, the formulation was able to suppress the MDA and ROS levels to a significant extent in human keratinocytes as well as the expression levels of MMP-2 and MMP-9. The encapsulation of EGCG in the transfersomes resulted in higher skin permeation and deposition of this flavonoid in the skin, compared with plain EGCG. Interestingly, the co-entrapment of HA in the formulation increased both the skin permeation and deposition of EGCG, thus demonstrating that this system constitutes a useful and effective EGCG cutaneous delivery vehicle, with synergistic antiaging and antioxidant benefits
[43].
Fang and colleagues assessed the possibility of using multilamellar phosphatidylcholine (PC) liposomes studied for topical and intratumor delivery administration of catechin, EC, and EGCG in nude mice
[44][45]. The authors showed that the inclusion of anionic species such as deoxycholic acid and dicetyl phosphate increased the encapsulation of the catechins and the permeability of the lipid bilayers. EGCG performed differently due to its higher lipophilicity. In addition, the authors reported an even higher EGCG encapsulation for deoxycholic acid-liposomes prepared in the presence of 15% ethanol as well as an increased catechin in vitro and in vivo skin permeation and deposition in basal cell carcinomas compared with both the free form and ethanol-free liposomes. This might be attributed to the fact that ethanol-enriched liposomes penetrate easily in the skin due to the increased elasticity conferred by the insertion of alcohol into the PC membranes. The results showed that optimization of the physicochemical features and composition of liposomes could control and improve the delivery of catechins. Moreover, the results suggested that the intratumor administration of liposomes might be an effective approach for the local treatment of solid tumors
[44][45].
Overall, there are several strategies that can be adopted to increase the solubility and subsequent bioavailability of flavonoids with therapeutic potential. Although much progress has been recently made, novel drug delivery systems suitable for an optimized topical application should continue to be explored
[21][46][47][48][49]. A summary of the therapeutic application of flavonoids and the different nanocarriers used to enhance their delivery to the skin is described in
Table 1.
Table 1. In vitro and in vivo studies using different nanocarriers for enhanced topical delivery of flavonoids to the skin.
| Flavonoid |
Nanoformulation |
Skin Model |
Therapeutic Application |
Ref. |
| Quercetin |
Solid lipid
nanoparticles |
Human skin |
Delay UVB radiation-mediated cell damage and necrosis |
[30] |
| Non-ionic emulsion with high lipid content |
Pig ear skin |
Inhibition of UVB-induced cutaneous oxidative stress and inflammation |
[4] |
| Anionic emulsion with low lipid content |
Pig ear skin |
Inhibition of UVB-induced cutaneous oxidative stress and inflammation |
[4] |
| Lecithin-chitosan nanoparticles |
Male Kunming mice |
Topical delivery system with a wide range of applications |
[28] |
| Lipid microparticles |
n.a. |
Enhance quercetin stability in topical formulations |
[27] |
Colloidal silica
emulsion |
Human skin |
Optimization of a formulation with enhance penetration into human SC |
[48] |
| Chitosan nanoparticles |
HaCaT cells |
Potential therapeutic agent for topical use against UVB radiation |
[29] |
| Penetration Enhancer containing Vesicles (PEVs) |
New born pig skin |
New formulation for dermal delivery of quercetin, with various therapeutic applications |
[31] |
| Polylactide nanocapsules; Multilamellar liposomes; Niosomes |
Subcutaneous injection in amistogote-infected hamsters |
Antileishmanial agent |
[3][49] |
| Liposomes with penetration enhancing vesicles (PEV) |
Female CD-1 mice |
Anti-inflammatory agent |
[5][49] |
| Lipid nanocapsules |
Acute monocytic leukemia cell line (THP1–1 cell) |
Antioxidant, anti-inflammatory agent |
[5][50] |
| Nanoparticle suspension |
Mice |
Antioxidant agent |
[5][41] |
| Catechins |
Multilamellar phosphatidylcholine-liposomes |
Female nude mouse (Balb/c-nu, 6–8 weeks old) |
Use of liposomes for the local delivery, including skin and tumor deposition, of polyphenols |
[3][43] |
| Ethanol enriched liposomes |
Female nude mouse (Balb/c-nu, 6–8 week) |
Antioxidant and chemopreventive activity |
[44] |
| Cream |
Iranian rabbits |
Wound healing effect |
[5][51] |
| Tansfersomes containing EGCG and hyaluronic acid (HA) |
HaCaT cells |
Synergize the UV radiation-protective ability of EGCG and HA along with imparting antioxidant and antiaging effects |
[5][42] |
| Genistein |
Nanoemulsion |
Pig ear skin |
New formulation for dermal delivery of genistein, with various therapeutic applications |
[3][52] |
| Kaempferol |
Submicron emulsions |
Sprague Dawley rat |
Promising vehicle for topical kaempferol application |
[33] |
| Resveratrol |
Solid lipid
Nanoparticles |
Porcine skin |
Protection from photodegradation |
[53] |
| Resveratrol + curcumin |
Lipid-core
Nanocapsules |
Human skin |
Increase skin delivery of resveratrol |
[54] |
| Niosomes |
Cell rabbit skin |
Increase skin delivery of resveratrol
Increased antioxidant activity |
[55] |
| Hesperetin, hesperidin |
Microemulsion |
Guinea pigs |
Whitening effect |
[5][56] |
| Topical matrix film |
Albino rabbits |
Release of hesperetin in posterior of eye |
[5][57] |
| Microemulsion based ointment |
Wistar rats |
Skin irritation |
[5][58] |
| Naringenin |
Gel |
HRS/J mice |
Antioxidant and anti-inflammatory agent |
[5][54] |
| Nanoparticles |
Wistar rats |
Photoprotective, antioxidant agent |
[5][54] |
| Apigenin |
Phospholipid phytosomes |
Albino rats |
Antioxidant agent |
[5][59] |
| Ethosomes |
Konmin mice |
Anti-inflammatory agent |
[5][60] |
| Anthocyanin |
Niosome gel |
Male Wistar rats |
Anti-inflammatory agent |
[5][61] |
| Luteolin |
Luteolin in olive oil |
ICR mice |
Anti-inflammatory agent |
[5][62] |
| Luteolin-loaded niosomes/Niosomal transgel |
Albino Wistar rats |
Treatment of arthritis |
[40] |
| Nanoemulsion |
C57BL/6 mice |
Growth promoting effect |
[41] |