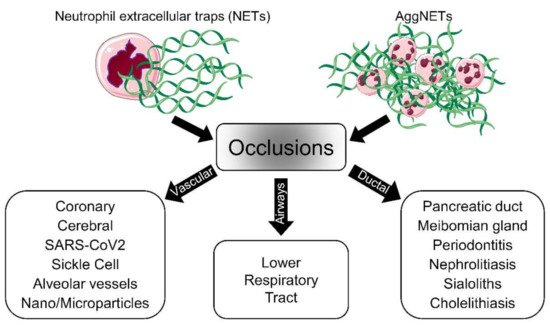
The discovery of neutrophil extracellular trap (NET) formation as a part of the defense mechanisms of the innate immune system has provided new insights into the pathologies of various diseases. Nowadays, NET formation is considered a double-edged sword, as NET remnants induce inflammation and aggregated NETs (aggNETs) reportedly occlude tubular structures like vessels or ducts. In this regard, elucidating the mechanism of NET-dependent occlusions is crucial for the development of new therapeutic approaches.
- neutrophil extracellular traps
- aggregation
- occlusions
- vessels
- ducts
1. Introduction
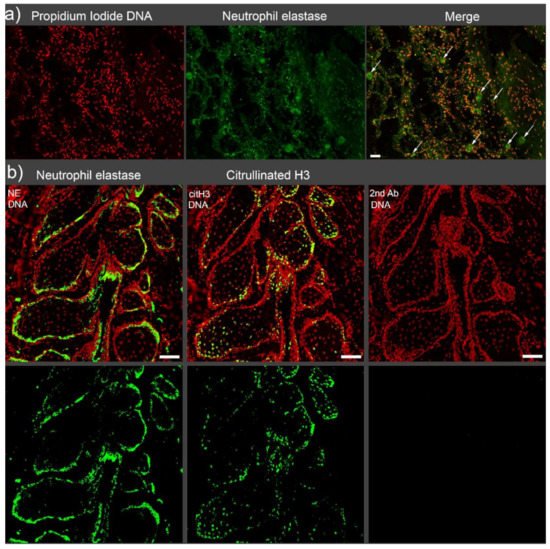
2. Vascular Occlusions
2.1. Vascular Occlusions in COVID-19
2.2. Coronary Occlusions
2.3. Cerebral Occlusions
2.4. Sickle Cell Disease
3. Airway Occlusions
4. Occlusions of Exocrine Glands and Ducts
4.1. Pancreatic Duct
The pancreatic duct, also named the duct of Wirsung, connects the pancreas and the intestine through the common bile duct. Its main function is to transport enzymes and bicarbonate, which aid digestion and neutralize the duodenal pH, respectively [75,76]. Occlusion of the pancreatic duct may cause pancreatitis. Occlusion of the ducts is directly proportional to the severity of pancreatitis and depends on the duration of the disease [75, 76].
NETs have been reported to directly induce trypsin activation, inflammation, and tissue damage in severe acute pancreatitis induced by retrograde taurocholate infusion [77]. However, in human acute pancreatitis, the direct cause of obstruction is not always found. Under physiological conditions, neutrophils are present in small amounts in the pancreas and enter the bicarbonate-rich pancreatic fluid, where they spontaneously form NETs [78]. In severe inflammatory situations, the resulting neutrophilia can produce excessive amounts of NETs in the pancreas. This results in large and sticky aggregates prone to occlude pancreatic ducts. Leppkes et al. reported that neutrophilia alone was the main driver of pancreatitis development in IL17 transgenic mice. In this case, excessive NET formation was immediately induced by high bicarbonate concentration in the pancreatic juice. The formed NETs tend to aggregate and occlude the ducts triggering focal acute pancreatic [79]. The reduced production and size of NETs and aggNETs in the pancreatic ducts of PAD4-KO mice prevented the development of focal pancreatitis [80, 81].
4.2. Meibomian Gland
The meibomian gland (MG) secretes a biological fluid (meibum) that contains a large amount and variety of lipids. Meibum is critical for maintaining a healthy ocular surface. Meibomian gland dysfunction (MGD) and the disruption of meibum homeostasis change the lipid content of the tear fluid [82, 83]. The lack of lipids in the tear film promotes hyperevaporation and tear hyperosmolarity.
As neutrophils are the first cells recruited to the sites of inflammation, NETs are discussed to drive the pathogenesis of MGD-related diseases, including dry eye disease (DED). The increased abundance of NETs in the tears and on ocular surfaces of patients with MGD and the positive correlation between the amount of NETs and disease severity has strengthened this suspicion [84]. Although aggNETs are known for their ability to resolve inflammation on the ocular surface [85], a recent study has shown that NETs are also implicated in MG terminal duct occlusions that trigger ocular surface inflammation [9]. This work has provided important new evidence to propose novel avenues in the treatment of MG occlusion-related disorders. In addition to the currently available medical treatments, such as antibiotics and non-steroidal and steroidal anti-inflammatory drugs, NET formation inhibitors should be included in the therapeutic arsenal of MGD [86].
4.3. Periodontitis (Periodontal Crevicular Occlusions)
The gingival crevice, also called the gingival sulcus, is defined as a narrow V-shaped space between the inner surface of the free gingival epithelium and the surrounding enamel of a tooth. Normally, its depth is 1–3 mm. The gingival epithelium continuously produces gingival crevicular fluid (GCF), which is eventually released into the oral cavity. It has been known for many years that the production of GCF and its composition change in inflammatory diseases like periodontitis. Therefore, GCF has been extensively studied as a diagnostic tool [87, 88]. Under physiological conditions, the minimal amount of GCF present in the gingival crevice flows into the oral cavity, turning the crevice into a kind of duct. Upon inflammation, this fluid transforms into a purulent exudate containing large amounts of NETs and neutrophils expressing CD177 [89, 90]. The exudate is extremely viscous due to the excessive amount of aggNETs. Vitkov and colleagues put forward the view that the NETs lead to obstruction of the periodontal crevice. The authors speculated that the formation of a periodontal abscess might result from NETs-induced cervical obstruction [6], supported by the overproduction of NETs and, impaired clearance of NETs remnants [91]. This hypothesis has not yet been experimentally proven. However, the crucial role of NETs in the initiation and progression of inflammatory periodontal diseases is surely worth investigating.
4.4. Gallstones
Obstruction of the biliary system is a common, serious, and painful condition and one of the leading causes of hospitalization with significant morbidity and mortality. The formation of gallstones in the gallbladder or bile ducts, also referred to as cholelithiasis, is the most common cause of biliary obstruction that results in biliary stasis. Gallstone-related biliary obstructions generate a high socioeconomic burden due to their high incidence, especially in developed countries [92, 93]. Until recently, gallstones were though to form simply due to supersaturation of cholesterol crystals. The contents of human gallstones were eventually investigated for NETs, as cholelithiasis is an occlusive condition [94]. After observing extracellular DNA and neutrophil elastase in gall sludge and gallstones, it was established that an intact NET formation capacity was necessary to form gallstones in a murine model of cholelithiasis. Basically, NETs were crucial for the initiation and progression of gallstone formation by promoting the aggregation of biliary cholesterol and calcium crystals. Furthermore, a positive correlation between the neutrophil/lymphocyte ratio and the severity of gallstone-induced pancreatitis has been reported in clinical settings [95]. However, formal clinical trials to inhibit NET formation in patients with recurrent cholelithiasis are still needed [96].
4.5. Sialoliths
Sialoliths, also known as salivary stones, are the most prevalent obstructive disease of the salivary glands, especially in middle-aged patients. Stone formation occurs mostly in the submandibular gland, with an incidence of more than 80 percent. This is followed by the parotid with 13 percent and by the sublingual and minor salivary glands with very low rates. The etiology of sialolith formation was initially considered to be a multifactorial interaction of calcium salts, organic and inorganic molecules, pH, and bacteria [97, 98]. Two conflicting reports claimed that inorganic materials [99] and organic components [100] were the main components of sialoliths. However, since the role of inflammation in sialoliths formation [101] and the presence of bacterial residues, including bacterial DNA and biofilms in their structures, have been demonstrated [102], NETs have gained attention as a possible etiological factor. Furthermore, since bacterial biofilm structures have been detected at the core of sialoliths, it has been hypothesized that biofilms are an initial step in the formation of sialoliths [103]. Recently, neutrophil recruitment and NET formation in the salivary system were suggested to initiate sialolithiasis. Indeed, evidence of NE activity associated with a high prevalence of extracellular DNA points to NETs as nidi for the formation of sialoliths [104]. The high content of bicarbonate in saliva strengthens this hypothesis as it facilitates the formation of NETs [78]. It is discussed that the interaction between neutrophils and the precipitated particulates leads to the growth of salivary stones. These results offer an alternative perspective to the “previously” proposed mechanisms of sialolithogenesis [105, 106].
5. Therapeutic Approaches to Prevent NETs-Driven Occlusions
In addition to their protective effects against pathogens, NETs contribute to the regulation of innate and adaptive immunity. In the case of persistent neutrophil activation, NETs form aggregates that contribute to the resolution of inflammation, reflecting the beneficial side of NET formation. Depending on where aggNETs are formed (e.g. vessels or ducts), these large and sticky aggregates might cause various types of obstructions. These occlusions drive the pathophysiology and increase the severity of many diseases, including COVID-19, chole- and sialolithiasis, dry eye disease, and probably many others. Therefore, it is critical to develop new effective therapeutic strategies aimed at either controlling the accumulation of NETs or improving their clearance at specific anatomic locations [26, 116]. NETs-inducing agents are diverse, and the formation mechanisms of NETs vary depending on these triggering agents.
Consequently, the mechanism of NET formation has to be explored for each individual condition, and then therapeutic strategies have to be developed in a condition-specific manner. NE, MPO, PAD4, and extracellular DNA are promising targets for suppressing excessive NET formation [11, 28] and are therefore currently the focus of drug development (Figure 3). Among them, inhibition of PAD4 and treatment with DNase1 are the most promising candidates for NETs-driven occlusions [91].
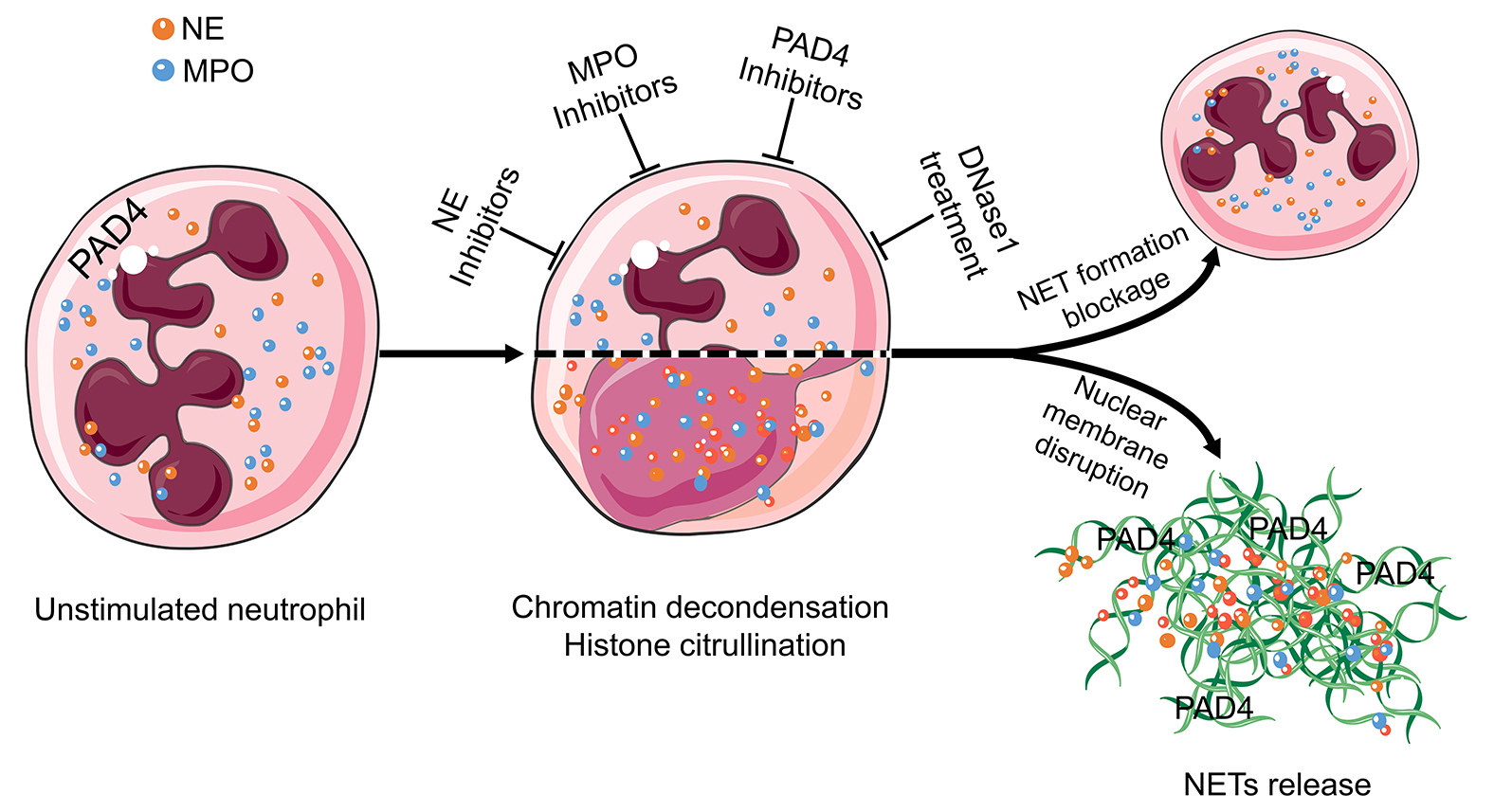
Figure 3 Targets in the treatment of aggNETs-driven occlusive pathologies. NE, MPO, PAD4, and extracellular DNA are promising targets for the suppression of excessive NETs formation. Enzymatic inhibitors of PAD4 and MPO have demonstrated its effectivity in preclinical settings of cholelithiasis, Meibomian gland dysfunction, and vasculitis. DNase treatment is effective at preventing death in severe sepsis and improving the symptoms in cystic fibrosis, bronchiolitis, and dry eye disease. The cartoons were modified from https://smart.servier.com accessed on 20 August 2021 in compliance with the terms of the Creative Commons Attribution 3.0 Unported License (CC BY 3.0). Abbreviations: MPO, myeloperoxidase; NE, neutrophil elastase; NET, neutrophil extracellular traps; and PAD4, peptidylarginine deiminase type IV.
5.1. Inhibition of PAD4 Reduces Formation and Size of NETs
Peptidylarginine deiminases (PADI) are a calcium-dependent enzyme family responsible for post-transcriptional deamination/citrullination. In this process, the positively charged arginine residues are converted into uncharged citrulline residues. The PADI family consists of five members. PAD4 is unique among them. It plays a role in the formation of NETs by ensuring the decondensation of chromatin [117]. This makes PAD4 a potential therapeutic target for the treatment of occlusive NETs-related diseases. The increased activity of PAD4 and the therapeutic potential of its inhibition have been previously reported in preclinical settings for the formation of thrombosis [22], acute pancreatitis [81], cholelithiasis [94], meibomian gland disfunction [9], lung injury [64], and sickle cell disease [56].
5.2. Deoxyribonucleases Dismantle NETs
DNases have been studied for therapeutic purposes in obstructive conditions. DNase1 cleaves DNA by breaking its phosphodiester bonds. This disrupts the structural integrity of NETs and reduces the sizes and amounts of aggNETs [118]. Disruption of NETs with DNase1 has been shown not only to prevent vascular occlusion [25] but also to recanalize the already occluded vessel [119]. Similar results have been obtained in crystal clots-driven arterial occlusion [24]. The improvement of ventilation after inhalation of DNase1 in acute RSV bronchiolitis has also been demonstrated [71]. DNase1 is already approved as an inhalation agent to reduce the viscosity of mucus in the lungs of patients with cystic fibrosis [74].
5.3. Inhibitors of Myeloperoxidase Reduce the Early Phases of NET Formation
One of the most important elements of NETs, especially in the early phase, is MPO. When neutrophils encounter danger signals, MPO enters the nucleus and drives chromatin decondensation, a crucial step in NET formation [120]. Inhibitors of MPO such as PF-1355 have been tested as NET formation blockers in small vessel vasculitis. In this context, PF-1355 prevented excessive NETs formation and reduced leukocyte infiltration [121], making it a good candidate for further studies trying to interfere with NET formation in occlusions. Furthermore, natural surfactants also have the ability to inhibit NET formation in vitro [122]. Therefore, natural surfactants bear the potential to be used as therapeutic agents in occlusive conditions.
6. Conclusions
Neutrophils and NETs participate in the initiation, pathogenesis, and resolution of various inflammatory conditions. In most cases where neutrophils are involved, significant collateral damage is expected. Therefore, robust regulatory mechanisms like apoptosis and NET formation have evolved to endow neutrophils with the ability to promote amelioration of initial inflammation. The clearance of apoptotic neutrophils by professional phagocytes triggers potent anti-inflammatory and regenerative responses [123, 124]. NET formation and aggregation actively limit the spread of pathogens and inflammatory mediators [7, 125]. Unfortunately, those anatomical locations carrying fluids or air through the body are prone to be occluded by NETs generating heterogeneous pathologies. Investigation of the prevention or resolution of such occlusions will provide new therapeutic opportunities for old, prevalent diseases.
This entry is adapted from the peer-reviewed paper 10.3390/cells10092208
