Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The emerging role of Cellular Retinoic Acid Binding Protein 1 (CRABP1) as a mediator of non-canonical activities of retinoic acid (RA) and relevance to human diseases.
- CRABP1
- retinoic acid
- neurodegeneration
- inflammation
 1. Introduction: Canonical and Non-Canonical Activities of All-Trans Retinoic Acid (atRA)
1. Introduction: Canonical and Non-Canonical Activities of All-Trans Retinoic Acid (atRA)
Vitamin A (also known as retinol) is an essential nutrient required for almost all physiological processes [1]. The profound effects of vitamin A are elicited mainly through atRA, as well as its various isomers. Through decades of studies, it has been established that atRA, as the principal active metabolite of vitamin A, executes its activities through binding to its nuclear receptors, RA receptors (RARs), which usually pair with Retinoid X Receptors (RXRs), can bind the cis isomers of atRA. These RAR/RXR pairs, in various combinations, act to regulate the transcription of numerous target genes that harbor RA response elements (RAREs) in their regulatory regions [2][3]. RAR/RXR pairs often also act together with other transcription factors to confer further specificity in the expression of target genes, resulting in the tight regulation of specific cellular processes such as proliferation [4], differentiation [5], apoptosis [6], and other physiological functions. These ultimately ensure the homeostasis of most organ systems/physiological processes [2][7]. Dysregulated RA signaling often leads to disease conditions [8][9][10]. These RAR-mediated activities of RA, which occur in the nucleus to regulate the execution of genetic programs, generally span an extended period of time (days to years) and are referred to as canonical activities of RA [11]. An extensive body of work has determined that the cellular retinoid-binding proteins (CRABPs) I and II facilitate these canonical activities of RA. Rigorous biochemical studies have characterized classical CRABP1 functions in RA binding, sequestration, and metabolism via cytochrome (CYP) P-450 enzymes [12][13], (reviewed in-depth in [14][15][16][17]) while CRABP2 is responsible for the transport of RA to the nucleus [14][15][18][19].
In 2008, a study first reported a novel activity (effect) of atRA that occurred rapidly (within minutes) to alter the protein phosphorylation status of a transcription factor TR2 in maintaining stem cell proliferation and stemness potential [20]. Subsequent studies [21][22][23] further documented similar activities of atRA that shared several features: (1) RAR-independence, (2) occurring in the cytosol without altering gene expression, and (3) rapid (typically within minutes) action. These novel activities of atRA were collectedly referred to as “non-canonical” and were later found to be mediated by the Cellular Retinoic Acid Binding Protein 1 (CRABP1) [22]. These CRABP1-mediated non-canonical activities of atRA were ultimately validated in careful studies of Crabp1 knockout (CKO) mice and cultures, which also revealed the physiological/disease relevance of CRABP1 [24][25][26][27][28][29][30][31][32][33].
Extensive molecular and cell biological studies have identified specific cytosolic signaling pathways that can be targeted by CRABP1 in a cell context-dependent manner. It is believed that CRABP1 functions as a signal integrator by forming various specific RA-regulated signaling protein complexes (signalsomes) in different cells to modulate specific cellular processes/functions.
2. CRABP1-Signalsomes
CRABP1 is the most highly conserved retinoid-binding protein among all the known binding proteins and nuclear receptors for retinoids. CRABP1 binds, specifically, atRA with a high affinity (<1 nM) [34][35][36][37]. Given its high affinity toward atRA and cytosolic distribution, CRABP1 has been proposed and shown to sequester the poorly soluble RA from the aqueous cytosolic environment [12][13][14][15][16][17][30]. This led to the belief that CRABP1 would function to control RA availability in the cell, which indeed was supported by several molecular studies, by altering the expression level of CRABP1, that documented subsequent changes in the expression of RA-responding genes [38][39]. As introduced earlier, CRABP1 could participate in RA metabolism by delivering RA to CYP P-450 metabolic enzymes and microsomes via protein-protein interactions and substrate channeling [15][40]. However, the physiological role of CRABP1 in mediating the newly observed, non-canonical activity has remained largely elusive. Only recently, studies of CKO mice and cultures in various physiological/pathological conditions (see the following section) began to shed light on multiple functional roles of CRABP1 in modulating specific cellular processes, which contributed to the “non-canonical” activity. The fact that CRABP1 is important for multiple signaling pathways is consistent with the extremely high conservation of its amino acid sequence across animal species. Figure 1 shows the reported amino acid sequence alignment of CRABP1 among five animal species including human [41], pig [42], rat [43], mouse [44], and bovine [45]. Importantly, there is only a single residue, at position 86, that is not conserved, with alanine in human and pig sequences and proline in mouse, rat, and bovine sequences (Figure 1).
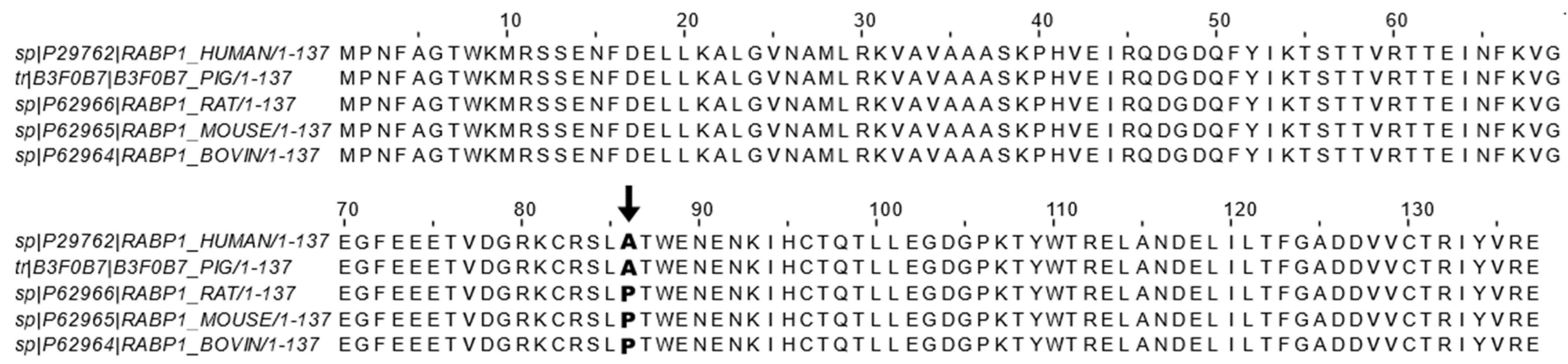
Figure 1. CRABP1 sequence alignment across mammals. Reported CRABP1 protein sequences from the Uniprot database of human (ID: P29762) [41], pig (ID: B3F0B7) [42], rat (ID: P62966) [43], mouse (ID: P62965) [44], and bovine (ID: P62964) [45] were aligned using the ClustalWS alignment algorithm in Jalview. Only the residue at position 86 (indicated by bold text and arrow ↓) is not conserved and exists either as an alanine (A) in human and pig sequences or as a proline (P) in bovine, rat, and mouse sequences.
The extreme conservation of CRABP1 during evolution would suggest important functional constraints. The evidence for this notion was obtained in careful studies of CKO mouse phenotypes (see later). Mechanistic details were provided in biochemical and cellular studies that first revealed specific context-dependent “binding partners” of CRABP1, which were rigorously defined according to at least two criteria: (a) specific and direct binding to CRABP1, which could be validated in vitro, and (b) forming specific cytosolic protein complexes that could be validated in vivo. Functional consequences of these CRABP1-containing protein complexes were each found to be capable of modulating certain specific cytosolic signaling pathways in a particular cell type. These CRABP1-containing protein complexes are therefore referred to as CRABP1-signalsomes. Currently, two types of CRABP1-signalsomes have been identified, which are discussed in the following sections.
2.1. CRABP1-MAPK (RAF-MEK-ERK) Signalsome in Stem Cells, Cancers
A specific Crabp1-signaling complex was first proposed after studying embryonal carcinoma (EC) and embryonic stem (ES) cells that were stimulated by a physiological concentration (10 nM) of atRA to modulate their proliferation/differentiation (reviewed in [46][47]). The initial study detected a very rapid (within minutes) response of these cells to atRA administration, which occurred in the cytosol and involved a mitogen-activated protein kinase (MAPK) pathway to modify target proteins for specific post-translational modifications [20][21][22][23]. This atRA-elicited signal was found to involve CRABP1, and could rapidly (within minutes) alter (dampen) the activity of the initiating kinase of the MAPK pathway, which is the rapidly Accelerated Fibrosarcoma (RAF) kinase and is a cell membrane-anchored kinase activated by the mitogenic signal Ras GTPase [48]. The MAPK kinase signaling cascade is comprised of Ras GTPase which activates RAF, then mitogen-activated protein kinase kinase (MEK), and then extracellular-signal-regulated kinase (ERK). Activation of this signaling pathway generally leads to cell proliferation and growth for stem/progenitor cells [48]. Through biochemical and molecular studies, it is now established that CRABP1 competes with Ras by directly interacting with RAF at its Ras-binding domain, thereby dampening MAPK signal propagation and ultimately modulating (reducing) cell proliferation of ES, EC, and neural stem cell (NSC) [22][25][29]. The proposed mechanistic model for CRABP1-MAPK signalsome is shown in Figure 2.
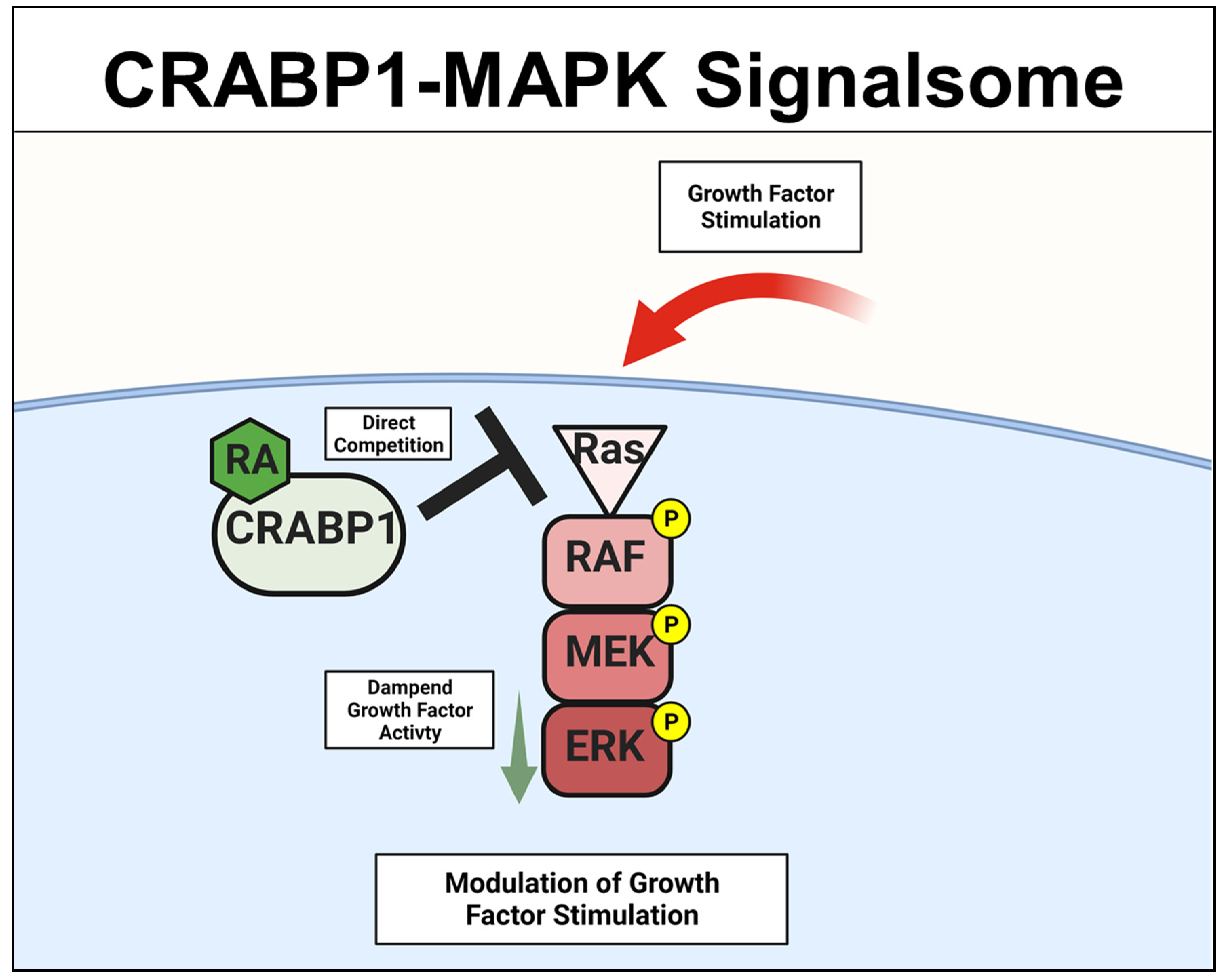
Figure 2. CRABP1-MAPK signalsome. The action of CRABP1-signalsome in growth-factor stimulated MAPK activity is mediated by its direct competition with Ras, resulting in dampened MAPK activation. CRABP1: Cellular Retinoic Acid Binding Protein 1, RA: retinoic acid, RAF: rapidly Accelerated Fibrosarcoma, MEK: mitogen-activated protein kinase kinase, ERK: extracellular-signal-regulated kinase.
To this end, the physiological/pathological relevance of CRABP1 is most evident in cancers. For instance, the CRABP1 gene has been reported as a tumor suppressor or an oncogene in animals and humans [15][49][50][51][52][53][54][55][56][57][58][59][60][61]. In comparing CKO and wild-type ESCs, as well as in gain- and loss-of-functional studies of cancer cell models, it was found that CRABP1 was involved in modulating cell cycle control [22]. By competing with Ras for forming complexes with RAF/MEK, atRA-CRABP1 dampened mitogen-activated ERK activity and suppressed cell cycle progression by expanding the G1 phase [22][29]. This supports the notion that CRABP1 can be a tumor suppressor. Additional evidence supporting a functional role for CRABP1 in stem cell proliferation was obtained from studying CKO mice that were found to have expanded NSC pools (as a result of enhanced NSC proliferation in CRABP1-deleted hippocampus), which was consistent with the CKO mouse behavior indicating improved memory function [25]. Importantly, the hippocampus is among the tissues where CRABP1 is most highly expressed, especially in the NSC-rich region of the dentate gyrus. Thus, CRABP1 can participate in the homeostatic control of the NSC pool in the brain. Readers are referred to an in-depth review of this CRABP1-regulated signaling pathway by Nagpal and Wei [62].
2.2. Crabp1-MAPK Signalsome in Metabolism and Immunity
Lin et al. first observed that CKO mice exhibited increased high-fat diet (HFD)-induced obesity and insulin resistance (IR), suggesting a protective role for CRABP1 against the development of metabolic disorders. A molecular study of CKO mice elucidated an underlying mechanism for this metabolic phenotype that, in normal adipocytes, CRABP1 could negatively regulate ERK activity to inhibit adipogenesis and adipose hypertrophy [28]. Therefore, CKO mice are more prone to HFD-induced obesity and IR. To this end, it has been reported that pharmacological doses of RA could inhibit adipogenesis and protect against obesity, and this was attributed, primarily, to RAR-mediated activities [63][64][65][66][67]. These recent studies of CKO models revealed CRABP1 as an additional player in mediating physiological activities of atRA regarding metabolic homeostasis and the maintenance of healthy adipose tissue [28].
In examining the systemic inflammatory status/potential of CKO mice, it was found that HFD-fed CKO mice all had increased systemic inflammation, indicated by invading immune cells in adipose tissue [28], increased inflammatory driver Receptor Interacting Protein 140 (RIP140) (gene name Nrip1) [68] in the blood [31], elevation in inflammatory cytokines, and significantly enhanced macrophage M1 polarization (unpublished). Previous studies also indicated that CKO mice had overall increased inflammation in the heart, indicated by increased cardiac fibrosis [26], and an altered anxiety and stress response in their HPA axis [32]. To this end, CRABP1 was found to be involved in exosome secretion from CRABP1-expressing neurons. Specifically, the RIP140-containing exosome population was significantly expanded in the blood and cerebral spinal fluid (CSF) of CKO mice, due to, in part, increased exosome secretion from CKO neurons [31]. Importantly, these neuron-derived RIP140-containing exosomes could be engulfed by macrophages to increase their inflammatory M1 polarization, thereby increasing systemic inflammation. This study, by monitoring the intercellular transfer of the inflammatory driver, RIP140, demonstrates exosome secretion as a potent means to transfer neuronal inflammation into systemic inflammation; mechanistically, this study identifies CRABP1 as an important regulator of exosome secretion from specific CRABP1-expressing neurons, which also involves the MAPK-ERK signaling in these neurons [31].
2.3. CRABP1-CaMKII Signalsome in Cardiomyocytes and Motor Neurons (MNs)
A different CRABP1-signaling complex was identified from studying deteriorated heart function of CKO mice [26][27], and their premature weakening in motor function [33]. The expression study confirmed CRABP1 expression in cardiomyocytes [26] (relevant to the CKO heart phenotype) and motor neurons (relevant to the CKO motor function phenotype) [33]. This signaling complex is comprised of CRABP1 and calcium-calmodulin-dependent kinase 2 (CaMKII), an enzyme critical to calcium signaling/handling and highly enriched in both cardiomyocytes [69] and neurons [70][71]. It is known that CaMKII regulates contraction in cardiomyocytes [69] and long-term potentiation in neurons [70][71], respectively. Both types of cells are highly dependent upon calcium homeostasis for their functions where CaMKII is a key mediator of calcium signaling [72]. All the CaMKII isoforms have a conserved architecture comprised of the kinase, regulatory, and association/oligomerization domains, and share the same activation mechanism through the binding of calmodulin to the calmodulin-binding domain (CaMBD) within its regulatory domain. CaMKII activation occurs when intracellular (Ca2+) increases and binds calmodulin. Ca2+-calmodulin then binds and activates CaMKII, which is often marked by phosphorylation at threonine 286/7 (T286/7), depending on the CaMKII isoform [73][74]. In vitro data showed that CRABP1competes with calmodulin by directly interacting with CaMKII at the CaMBD [26][27]. Therefore, CRABP1 could dampen Ca2+/Calmodulin activated CaMKII activity. Since over-activation of CaMKII is a major trigger of the death/damage of cardiomyocytes [75] and neurons [76], by dampening CaMKII over-activation, CRABP1 can play a protective role in maintaining the health of both the heart and neurons. These are elaborated on in the following section. The proposed mechanistic model for CRABP1-CaMKII signalsome is shown in Figure 3.
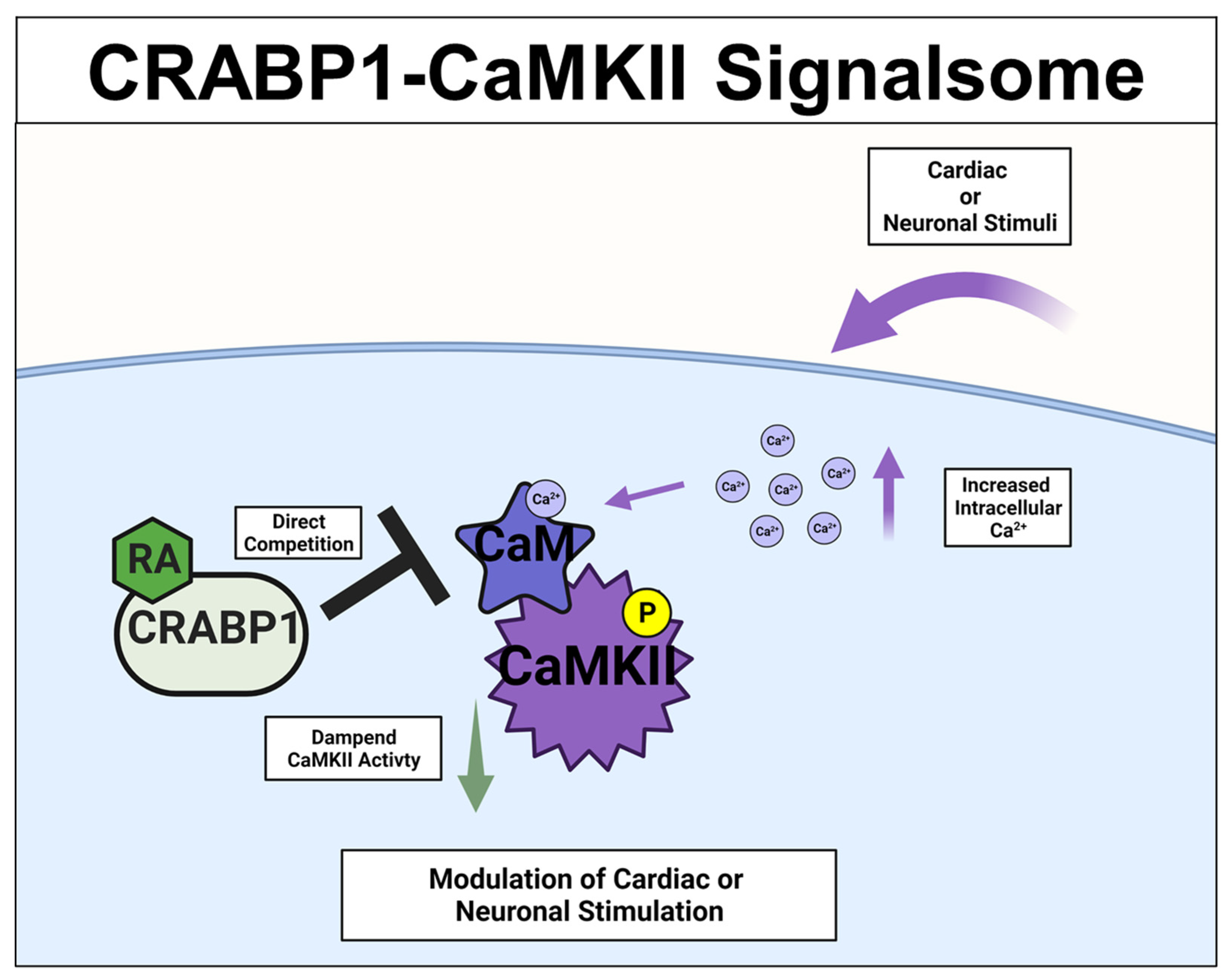
Figure 3. CRABP1-CaMKII signalsome. Upon cardiac or neuronal stimulation and subsequent intracellular Ca2+ increase to activate CaMKII, CRABP1 directly competes with calmodulin (CaM) to dampen CaMKII enzyme activity to ultimately modulate cardiac and/or neuronal stimulation. CRABP1: Cellular Retinoic Acid Binding Protein 1, RA: retinoic acid, CaMKII: calcium-calmodulin-associated dependent kinase 2.
This entry is adapted from the peer-reviewed paper 10.3390/nu14071528
This entry is offline, you can click here to edit this entry!
